Table of Contents
As you may already know, the concentration of reactants decreases while the concentration of products increases as a given reaction proceeds.
But are the changes in the concentration of reactants and products continuous?
After a while, the concentrations of reactants and products no longer change, meaning that the reaction has reached the state referred to as a chemical equilibrium.
If the chemical equilibrium results from two active and opposing reactions, the equilibrium that the system reaches is referred to as a dynamic equilibrium. In the case of dynamic equilibria, there is no observable change present in a reaction; However, the system is in constant motion, meaning that the reactants are continuously transformed into products, and products are transformed into the reactants. The system is considered a closed system (sealed container), meaning that there are no components added or removed from it.
To note the dynamic equilibrium in a chemical equation, you should simply use two opposing arrows between the reactants and the products.
The study of equilibria shows how far the given reaction goes.
The most common example of dynamic equilibrium is the transition from liquid to vapour phase and vice versa. Heat is needed for liquid to vaporize and transform to the vapour phase, while if the vapour is condensed, the same amount of heat is released.
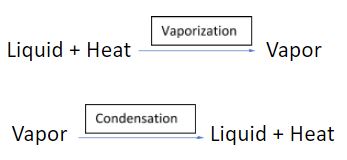
Since the same quantity of heat is released during the vapour-to-liquid-phase transition as it is needed for the liquid-to-vapour-phase transition, the two reactions above can be written in the following manner:
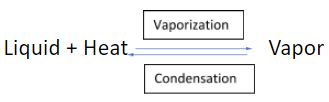
The only thing that we change is that we add an arrow facing in the opposite direction.
Similarly, various chemical reactions also proceed in simultaneous processes with opposing directions. These two opposing reactions are referred to as forward and back (also referred to as reverse) reactions. In such cases, the reaction is said to be a reversible reaction. Common examples of reversible reactions are various biochemical reactions (enzymatic reactions) occurring in different living organisms.
But in some cases, the reaction between the products does not produce the initial reactants. In other words, such a reaction is said to be an irreversible reaction.
In the case of irreversible reactions, we just use a single arrow pointing to the products. Examples of such reactions include the combustion of hydrocarbons, the products of which are carbon dioxide and water (CO2 and H2O). Obviously, you will not get the hydrocarbon as a product if you react CO2 with H2O.
Continuing with reversible reactions, the following equation can be used as a general one:

Equilibrium State
Every time you see a reaction with two arrows facing in opposite directions, you can assume the following two things:
- The chemical has already reached or is capable of reaching an equilibrium state.
- The equilibrium state for a reaction can be reached from both directions, meaning that the equilibrium may be reached by adding either A to B or C to D.
In any reversible reaction at equilibrium, forward and back (reverse) reactions have the same reaction rate, and the concentrations of reactants, as well as products, remain constant during the reaction.
The following figure shows the relationship between the molar concentration of products and reactants and elapsed time:
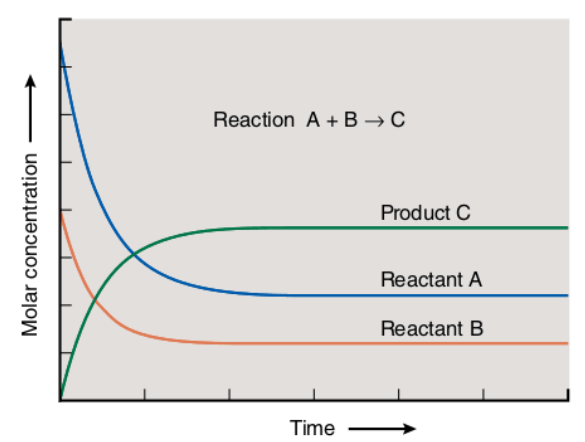
As you can see, the blue curve corresponds to reactant A, while the red curve indicates reactant B. Product C is represented with the green curve.
At the beginning of a reaction “A+B????C,” the concentrations of reactants A and B were decreasing, while the concentration of product C was increasing. After some time, the concentrations of reactants and products remain constant. This is the point when a chemical reaction has reached its equilibrium state.
In such reactions, the use of reactants to produce products slows the rate of the forward reaction. As more products are formed, the rate of back (reverse) reaction increases. After some time, the forward and back (reverse) reaction rates remain the same, meaning that the system is at an equilibrium state.
Note that the concentrations of the reactants and products remain the same, but it does not necessarily mean that [reactants] = [products]. The concentration of reactants might be more than the concentration of products. We only state that the concentration of reactants and products remains constant (do not change) after a while.
[ ] – the brackets like these just indicate the concentration.
[reactants] – indicates the concentration of reactants
[products] – indicates the concentration of products
The rate of a reversible reaction depends on several variables, including the concentration of the reactants. Evidently, the concentration of the reactants is the highest at the beginning of a reaction; Therefore, the rate of the forward reaction is also the highest at this point. As the concentration of reactants decreases, the speed of the forward reaction also decreases. As soon as the concentrations of reactants and products reach the point when they no longer change, the system is at the equilibrium state. As we already mentioned above, the reactions do not stop. They simply continue to happen in the equilibrium phase, meaning that forward and reverse reactions are occurring at the same time without changing the rate or concentration of components.
Exothermic and Endothermic Reaction
Another concept that you should consider in the case of reversible reaction is that if the forward reaction is exothermic, the reverse reaction will be endothermic.
If the reaction is exothermic, the energy is released in the form of heat. Therefore, the ΔH value of a reaction is negative.
If the reaction is endothermic, the energy is absorbed by the system. Therefore, the ΔH value of a reaction is positive.
Homogeneous and Heterogeneous Equilibrium
If all components of a reaction are in the same phase, the equilibrium that is reached in a reaction is referred to as homogeneous equilibrium.
On the contrary, if the species involved in a chemical reaction are in different phases, equilibrium is stated to be heterogeneous equilibrium.
Le Chatelier’s Principle
Used to predict how the changes in temperature, pressure, and concentration affect the position of equilibrium in a reaction. More specifically, to which direction will the equilibrium shift if we change any of the variables mentioned above.
Note that if the position of equilibrium was affected by altering temperature, pressure, or concentration of reactants and products, it could not be influenced by the presence of a catalyst. Catalyst can only decrease the activation energy and increase the speed of a given reaction. It cannot shift the equilibrium in any direction.
To quantitatively determine the number of products relative to reactants at equilibrium, we use the equilibrium constant – Keq.
Summary
Terms and concepts defined throughout the article are summarized in the table provided below:
| Chemical Equilibrium | The state when the concentrations of reactants and products no longer change. |
| Dynamic Equilibrium | There are no observable changes in a reaction, but the system is in constant, dynamic motion in both directions. Reactants are constantly transformed into products and products are constantly transformed into reactants as well. |
| Reversible Reaction | Reactions that proceed into both forward and reverse directions simultaneously. |
| Irreversible Reaction | Reactions that only proceed in a forward direction. |
| Homogeneous Equilibrium | A reaction in which the reactants and products are in the same phase. |
| Heterogeneous Equilibrium | A reaction in which reactants and products are in different phases |
| Le Chatelier’s Principle | Predicts how the changes in temperature, pressure, or concentration of reactants and products affect the position of equilibrium in a reaction. |
| Equilibrium Constant | The value is derived using the concentrations of reactants and products in a reaction at an equilibrium state. |
Frequently Asked Questions
What is dynamic equilibrium?
Dynamic equilibrium is a state of reversible reaction when the concentration of reactants and products becomes constant. It means that the rate of the forward reaction becomes equal to the rate of the reverse reaction at this stage.
How is dynamic equilibrium achieved?
Initially, the concentration of reactants in a chemical reaction is high, and the forward reaction rate is greater. The concentration of reactants and rate of the forward reaction decreases with time while the concentration of products and rate of reverse reaction increases with time until both reactions proceed at the same rate to establish the equilibrium.
What is the difference between homogenous and heterogeneous equilibrium?
If all the reactants and products are in the same phase, equilibrium is homogenous. On the other hand, if the components of a reaction are in different phases, equilibrium is heterogeneous.
Is dynamic equilibrium possible in irreversible reaction?
The irreversible reaction involves a forward reaction only. It doesn't have a reverse reaction, so dynamic equilibrium isn't possible in an irreversible reaction.
References:
OpenStax College. (2015). “Chemistry OpenStax College.” Retrieved from: http://cnx.org/content/col11760/latest/
If you like what you read and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





