Table of Contents
Key Information & Summary of Covalent Bonding
- A covalent bond is formed when two atoms share one or more pairs of electrons.
- Covalent bonding occurs when the electronegativity difference between elements (atoms) is zero or relatively small.
- Bonds form if there is an overlap of two atomic orbitals.
- The covalent bonds in a polyatomic ion can be represented using the Lewis formulation.
- Covalent compounds have lower melting and boiling points than ionic compounds
- Covalent compounds are soluble in nonpolar solvents, such as hexane, C6H14, and carbon tetrachloride, CCl
- Covalent bonds are non-polar in mononuclear diatomic molecules and polar in heteronuclear molecules depending on the difference in electronegativity between the different atoms.
We will start considering the simplest covalent bonding example, the union of two hydrogen atoms to constitute the diatomic molecule H2. An isolated hydrogen atom is characterized for having the ground state electron configuration 1s1, when the probability density of the single electron is distributed spherically distributed around the hydrogen nucleus. As two hydrogen atoms get closer to each other, both electrons feel the attraction by its original nucleus but also by the nucleus of the other hydrogen atom approaching. When the two electrons possess spins with opposite direction so according to the Pauli principle they are allowed to occupy the same orbital, both electrons can coexist in the same region between the two nuclei, because they are both feeling the attraction for both nuclei.
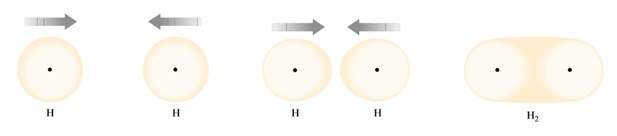
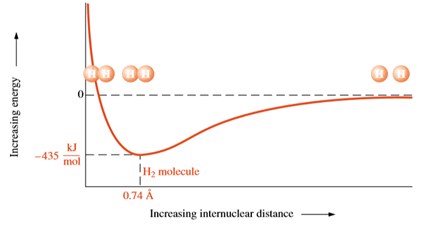
A single covalent bond is formed when electrons are shared between two atoms as the two orbitals overlap in a way that both electrons are both hydrogen atoms. In this case, each hydrogen atom has the helium configuration 1s2. The bonded atoms are always in state of lower energy and therefore more stable than the isolated atoms as the following figure shows an energy versus distance plot. As the distance between the two atoms is reduced the positively charged nucleus increase a repulsion force on each other. At a particular distance, when the energy is minimum, the most stable state occurs at an energy of 435 kJ/mol at 0.74 Å, which corresponds to the most stable configuration between two hydrogen nuclei in an H2 molecule. As the separation between nuclei gets larger, the repulsive forces diminish at a lower rate than the attractive forces resulting in a net attraction with a negative value of energy. On the other hand, at smaller separations, repulsive forces increase more rapidly than attractive forces resulting in net increasing repulsive force with positive value of the interaction energy.
This type of bond is common in other pairs of non-metal atoms sharing electron pairs so that each atom attains a more stable electron configuration close to the nearest noble gas which results in a more stable arrangement for the bonded atoms. Typical covalent bonds can share two, four, or six electrons implying one, two, or three pairs of electrons, usually named as simply single, double, and triple bonds
Either in a Lewis representation, a covalent bond is written as a pair of two dots between the two atom symbols or by a dash symbol connecting them. Here three examples of Lewis diagrams for the formation of H2, F2 and HF are shown;
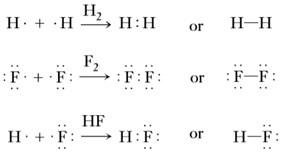
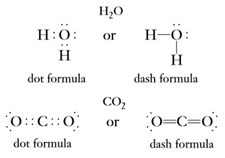
In H2O or CO2 two of six balance electrons of an oxygen atom are used in covalent bonding; the one valence electron of each hydrogen atom is used in covalent bonding.
The covalent bonds in a polyatomic ion can be represented using the Lewis formulation. The ammonium ion, NH4+, shows only eight electrons in its valence even though there is a total of nine (five electrons from the N atom and 4 for each the H atoms, for a total of 5 + 4(1) = 9 electrons). The NH4+ ion, with a charge of 1+, has one less electron than the sum of the total electrons from the original atoms achieving the noble gas configuration and can be represented with Lewis dot diagrams as it follows.
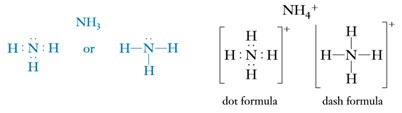
It is important to note that Lewis dot formulas only give information about the number of valence electrons, the number and type of bonds that facilitate the union of the atoms. They do not show any information regarding three-dimensional shapes of molecules and polyatomic ions.
Bond polarity
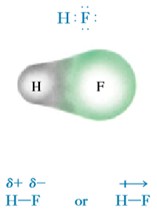
Covalent bonds can be either polar or nonpolar. Electronegativity δ is defined as the tendency of an atom to attract electrons to itself in a chemical bond. If we consider the H2 molecule where both H atoms have the same electronegativity then the shared electrons are equally attracted to both hydrogen nuclei and therefore both electrons spend equal amounts of time near each nucleus. This translates into having the electron density symmetrical about a plane that is perpendicular to a line between the two nuclei. This is generally true also for other homonuclear diatomic molecules, such as H2, O2, N2, F2, and Cl2, because the two identical atoms have identical electronegativity. For that reason, in general, the covalent bonds in all homonuclear diatomic molecules are nonpolar. However, when we consider covalent bonds between atoms with different electronegativity, the separation of charge in a polar covalent bond creates an electric dipole. We expect the dipoles in the covalent molecules HF, HCl, HBr, and HI to be different according to the different electronegativity of F, Cl, Br, and I.
Covalent compound characteristics
In comparison with ionic compounds, most covalent compounds possess low melting points and boiling points due to their weak inter-molecular interaction despite of showing strong forces inter-atom with respect to ionic bond. They usually also present lower enthalpies of fusion and vaporization and they have a tendency to be more flammable. In aqueous solution, covalent bonds do not conduct electricity.
Here are examples of covalent bonds and compounds:
PCl3 – phosphorus trichloride
CH3CH2OH - ethanol
O3 - ozone
H2 - hydrogen
H2O - water
HCl - hydrogen chloride
CH4 - methane
NH3 - ammonia
CO2 - carbon dioxide
Read more about types of chemical bonds
Frequently Asked Questions
What do you mean by covalent bond?
A covalent bond is a strong bonding force between two atoms established when they mutually share electrons to get stability. The bond between two fluorine atoms in F2 is purely covalent and formed by mutual electron sharing.
Why is polarity induced in a covalent bond?
A covalent bond becomes polar if it is formed between two atoms having an electronegativity difference. An electronegative atom attracts the bonding electron pair more strongly. As a result, δ− charge is induced on it. The other atom gains δ−+ charge.
Is water ionic or covalent?
The electronegativity difference makes hydrogen δ+ and oxygen δ-, so water is a polar covalent molecule.
What are the physical properties of covalent compounds?
Covalent compounds have comparatively low melting and boiling points and don't conduct electricity. They usually dissolve in non-polar organic solvents such as hexane and carbon tetrachloride.
References and further readings:
Kenneth W. Whitten-General Chemistry - Textbook Only-Cengage Learning (2000)
https://www.britannica.com/science/covalent-compound





