Table of Contents
Chemical bonding deals with the study of formation of compounds out of elemental forms of atoms. Various forms of bonding may be classified into the following types.
Ionic Bonding:
The other name of Ionic bonding is electrovalent bonding; the molecular linkages are formed by the electrostatic attraction among oppositely charged ions. The attraction phenomenon is influenced by the formation of bond between molecules with the help of complete valence electron transfer from one atom to another. The atom which loses electron(s) are positively charged and named as cation and the one that gains electron(s) are named as anion which have negative charge. The schematic representation of NaCl compound which exhibits Ionic bond is represented below:
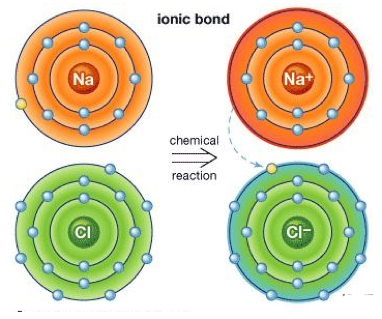
The best ionic bonding can be seen in the combination of non-metals, alkali and alkaline-earth metals. In molecular formation, like charges gets repulsed and those being oppositely charged, gets attracted in which each positive ion is surrounded by negative ions in attraction forming a net zero balance of ions for the stable ionic compound. The ionic bonds are also referred as extreme form of covalent bond, which happens only during a great difference in electronegativity of the participating atoms.
Covalent and Metallic Bonding in Chemical Bonding:
The sharing of electron pair among two atoms results in interatomic linkage termed as covalent bonding where the electrostatic occurs between the atoms having similar electronegativity resulting in binding process. Less the difference in the electronegativities, more is the strength of the covalent bond. A covalent bond is expected to form the bonding with lowest energy. The schematic representation of the covalent bond is shown below:
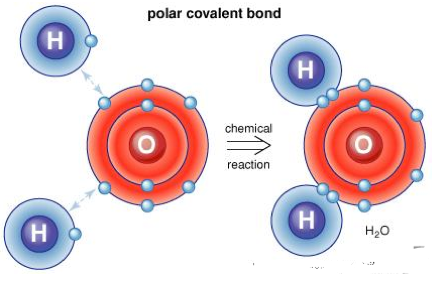
The covalent bonds can also be formed other than in organic compounds. Inorganic substances like water, chlorine, ammonia, nitrogen and hydrogen also exhibit covalent bonding. The covalent bonds in the molecules are represented by solid lines and examples are shown below.
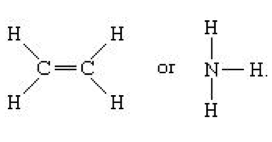
The single line represents single bond, two lines for double bonds, three lines for triple bonds which are represented symbolically as . The metallic bonding not being like ionic or covalent bonding, it is formed by the attraction of valence electrons and metal ions. In simple representation, many electrons surrounding the metal ions acts as glue in bond formation termed as metallic bonding. The loosely bound electrons forming a “sea” of floating valence electrons is the characteristic of metallic bonding. This very nature accounts for the highly electrical conductivity to metals.
The atoms in solid state like ionic bonded molecules arrange in patterns of order termed as lattice which is the space of minimum energy stored. The ionic lattice forms in ionic bonding while in covalent bonds a bigger lattice structure is formed and molecular lattice is formed in intermolecular bonding. In general, there are fourteen types of lattice commonly named as Bravais lattice and the types of lattice formed in atoms and molecules are:
- Cubic crystal with at all axes
- Hexagonal with four crystallographic axes forming at three horizontal axes
- Tetragonal with one perpendicular axes and other two in
- Rhombohedral in 3D similar to cubic with
- Monoclinic with three unequal axes where vertical and forward facing forms oblique angle and ortho axis is formed with two perpendicular axes to the horizontal faced axis with
Key Points:
- Ionic bonds are formed by complete transfer of valence electrons from one atom to another, between atoms having the greatest difference in their electronegativities.
- Covalent bonds are formed by sharing of valence electrons, between atoms having minimal difference in electronegativities.
- Metallic bonding occurs between atoms within a metal and has a “sea” of floating electrons on the surface.
- In the solid state, the ionic, covalent and metallic bonding, all are arranged in definite geometrical shapes which are known as lattices.
The measurement of the atoms tendency to attract the bonding pair of electrons are termed as electronegativity and the formal charge among the atoms / molecules represents its resonance state. The tool used to measure electronegativity of the atoms / molecules is the Pauling scale where least electronegative atoms are Caesium and Francium with the value of 0.7 and the most electronegative atom is Fluorine with 4.
The polar and non-polar bonding of the molecules are determined using electronegativity values, in polar bonds the one of the molecule is slightly positive and other end of the molecule is slightly positive favouring the dissolution of the molecule where as in non-polar molecules the charge in both the ends are not balanced it exists in uneven distribution results in non-dissolution of the atoms / molecules.
There are three main points about electronegativity
- The non-polar covalent bond exists when no electronegativity difference occurs.
- Only a small electronegative difference results in the formation of polar covalent bonds.
- The ionic bond results from the large electronegative difference.
The existence of molecular and ionic shapes occurs by the repulsive and attractive forces that exist within molecules and ions and generally accepted view is “The shape adopted is the one which keeps repulsive force to a minimum”. The types of molecular shape existence are listed below with the appropriate bond angle reference,
- Linear with 2 bond pairs of bond angle
- Trigonal planar with 3 bond pairs of bond angle
- Tetrahedral with 4 bond pairs of bond angle
- Trigonal bipyramidal with 5 bond pairs of bond angle combination
- Octahedral with 6 bond pairs of bond angle
Key points:
- Electronegativity of atoms determines the nature of chemical bond formed between them.
- Attractive and repulsive forces operating within molecules and ions determine their shapes.
Frequently Asked Questions
What is a chemical bond?
A chemical bond is the force of attraction between different atoms in a molecule.
Why is chemical bonding important?
When a bond holds two or more atoms together, new substances (compounds) are formed. Our life depends upon hundreds of compounds such as proteins, carbohydrates, fats, water, oxygen, etc. Without the existence of compounds, life becomes impossible.
How is a chemical bond formed?
Chemical bonds are firmed either by sharing electrons or by the complete transfer of an electron from one atom to the other.
Which bond is stronger, Ionic or covalent?
A covalent bond formed by mutual electron sharing is stronger than an ionic bond.
References:
- Gary L. Miessler and Donald A. Tarr, Inorganic Chemistry (4th Edition).
- https://www.khanacademy.org/science/chemistry/chemical-bonds





