Table of Contents
Key Facts & Summary
- Atoms are made of neutrons, protons and electrons.
- Neutrons and protons are situated in the nucleus, while the electrons orbit around the nucleus.
- Isotopes are versions of an element with a different number of neutrons.
- The number of protons for different isotopes is the same and does not change.
- Some isotopes are radioactive, others are stable and never decay or decay very slowly.
Introduction Atomic Structure & Isotopes
All substances are made up from one or more different kinds of simple materials known as “elements.” Among the common elements, for example, are the gases hydrogen, oxygen, and nitrogen; the solid nonmetals carbon, sulfur, and phosphorus; and various metals, such as iron, copper, and zinc.
The smallest part of any element that can exist, while still retaining the characteristics of the element, is called an “atom” of that element. Thus, there are atoms of hydrogen, of iron, of uranium, and so on, for all the elements. The hydrogen atom is the lightest of all atoms, whereas the atoms of uranium are the heaviest of those found on earth.
Heavier atoms, such as those of plutonium, also important for the release of nuclear energy, have been made artificially by human. Frequently, two or more atoms of the same or of different elements join together to form a “molecule.”
Atom structure
Every atom consists of a relatively heavy central region called nucleus, surrounded by a number of very light particles known as “electrons.” More than 99.94% of an atom's mass is in the nucleus. The atomic nucleus is formed by a definite number of fundamental particles, called “protons” and “neutrons.” (Figure 1)
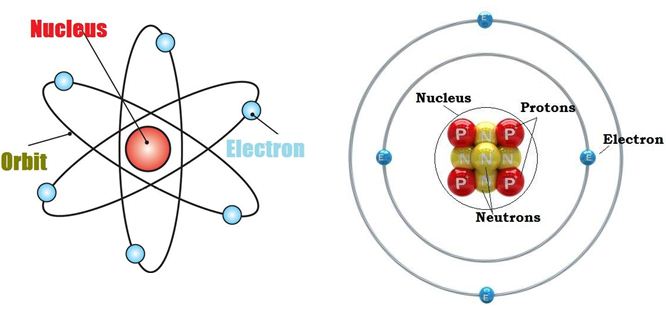
Fig 1.Graphic representation of an atom. Particles of interests are highlighted. (www.science.com, www.chemistryinfo.blogspot.com)
These two particles have almost the same mass. In fact, the mass of a proton or neutron is 1.66 e -24 grams or one AMU (atomic mass unit). The mass of an electron is 9.05 e-28 grams. This number is a billionth of a billionth of a billionth of a gram. Protons and neutrons differ in the respect that the proton carries a unit charge of positive electricity whereas the neutron, as its name implies, is uncharged electrically, i.e., it is neutral. The protons confer to the nucleus a positive charge, which is perfectly balanced by the number of electrons present in the orbits of the atoms, therefore, the latter is overall neutral.
Isotopes
The essential difference between atoms of different elements lies in the number of protons in the nucleus; this is called the “atomic number” of the element. Hydrogen atoms, for example, contain only one proton, helium atoms have two protons, uranium atoms have 92 protons, and plutonium atoms 94 protons.
Although all the nucleus of a given element contain the same number of protons, they may have different numbers of neutrons. The resulting atomic species, which have identical atomic numbers but which differ in their masses, are called “isotopes” of the particular element. The term isotope is formed from the Greek roots isos (ἴσος "equal") and topos (τόπος"place"), meaning “the same place” . Each atomic number identifies a specific element, but not the isotope.
Stable isotopes are generally defined as non-radioactive isotopic elements that do not decay over time to their daughter isotope. Radioactive isotopes may also be classified as stable isotopes when their half-lives are too long to be measured (in term of human life). These elements can often be found to occur in nature and include isotopes of carbon, nitrogen, hydrogen, oxygen, noble gases and metals.
Each isotope of a given element is identified by its “mass number,” which is the sum of the numbers of protons and neutrons in the nucleus. For example, the element uranium, as found in nature, consists mainly of two isotopes with mass numbers of 235 and 238; they are consequently referred to as uranium-235 and uranium-238, respectively.
The mass number is usually given in the upper left side of an element symbol.
For example, the isotopes of hydrogen may be written: 1H, 2H, 3H (hydrogen, deuterium and tritium respectively).
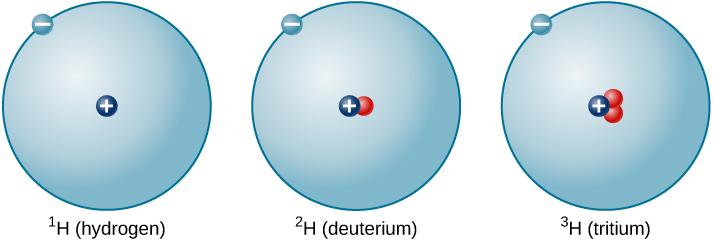
Fig 2. Graphic representation of isotopes of hydrogen. Dark blue: protons. Red: neutrons. Light blue: electrons. (https://phys.libretexts.org/TextBooks_and_TextMap)
Stable isotopes are generally defined as non-radioactive isotopic elements that do not decay over time to their daughter isotope. Radioactive isotopes may also be classified as stable isotopes when their half-lives are too long to be measured (in term of human life). These elements can often be found to occur in nature and include isotopes of carbon, nitrogen, hydrogen, oxygen, noble gases and metals.
Each isotope of a given element is identified by its “mass number,” which is the sum of the numbers of protons and neutrons in the nucleus. For example, the element uranium, as found in nature, consists mainly of two isotopes with mass numbers of 235 and 238; they are consequently referred to as uranium-235 and uranium-238, respectively.
Example of Isotopes
Carbon 12 and Carbon 14 are both isotopes of carbon: one with 6 neutrons and one with 8 neutrons (both with 6 protons). Carbon-12 (or 12C) is a stable isotope, while carbon-14 (or 14C) is a radioactive isotope (radioisotope), which t loses its extra neutrons and becomes 12C. The loss of those neutrons is called radioactive decay. That decay happens regularly in time. For carbon, the decay happens in a few thousand years. Some elements take longer, and others have a decay that happens over a period of minutes. Archeologists are able to use their knowledge of radioactive decay when they need to know the date of an object they dug up in a process called carbon dating.
Iodine-131 (or 131I) is an isotope of iodine, because it contains a different number of neutrons from 127I, therefore 131I has 4 more neutrons. 131I has been found useful in radiation treatments for thyroid cancer treatment.
Tritium (or 3H) is an isotope of hydrogen and is used to make things such as clock faces and wristwatches glow in the dark. Tritium provides an extremely bright self-activated and self-sustaining light source that will stay bright throughout the night and has a life span of twenty years. (Fig. 2)
Read more about Atomic Structure and Periodic Table
Frequently Asked Questions
What is the basic structure of an atom?
An atom is made up of three basic subatomic particles; protons neutrons and electrons. Protons and neutrons reside in the nucleus of the atom while electrons revolve around it. In addition, there are about 100 other subatomic particles that have been discovered so far.
What are isotopes?
Isotopes are atoms of the same element having the same atomic number but different mass numbers due to the difference in the no. of neutrons present in the nucleus. Hydrogen has three isotopes protium, deuterium and tritium having 0,1 and 2 neutrons respectively.
What is radioactive decay?
Radioactive decay is a spontaneous process by which nuclei of unstable elements undergo decay by emitting alpha, beta or gamma particles and become stable. In radioactive decay, the identity of an element changes with the decay.
What are the uses of radioactive isotopes of carbon and iodine?
Carbon-14 (a radioactive carbon isotope) is used in carbon dating or radioactive carbon dating to measure the age of the fossils. A radioactive isotope of iodine (I-131) is used in goitre treatment.





