Table of Contents
Introduction to amount of substance:
The universe houses all the material and chemistry is the science that evolves by experimentation and careful observation on these materials. This includes not only qualitative ideas, but also rigorous quantitative measurements. In order to understand the nature of the materials, we need to identify the units which make up those materials. In chemistry, these are called atoms – the basic units of an elemental form, and molecules – basic units of compounds. To designate the proportions of atoms in a chemical compound, the term used is “chemical formula”. These chemical formulae can further be classified into three classes - empirical formula, molecular formula, and structural formula. Empirical formula is the formula of a compound expressed in terms of the simple ratio of number of each element present, while the molecular formula represents the nature of the molecule and exact number of each atoms. The structural formula represents the mode of bonding in the molecule.
Read more about Formulae Equations and Amount of Substance
Structural Formula
Let’s take some examples:
Two compounds A and B have empirical formulae BH3 and C6H12O2. It is a simple ratio of elements in each. It is known from the observed properties, that A exists as a dimer i.e. it has a molecular formula B2H6. The compound B can exist in numerous forms like 3-(prop-2-en-1-yloxy)propan-1-ol, 2H-3,4,5,6-tetrahydropyran-4-ylmethan-1-ol, 3-methyl valeric acid and many more. Each of these are represented as the molecular formula C6H12O2. Hence, in this case, the molecular formula is same as the empirical formula. It must be noted that a single empirical and/or molecular formula represents all the possible isomers of a given compound.
Now let’s move on to the structural formula. It is the most clear representation of how the atoms are arranged in a molecule.
The structural formula of A
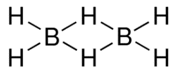
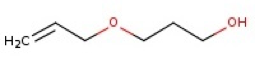
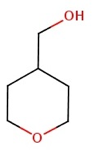
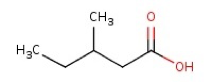
The structural formulae of some of the isomers of B
3-(prop-2-en-1-yloxy)propan-1-ol
2H-3,4,5,6-tetrahydropyran-4-ylmethan-1-ol
3-methyl valeric acid
Key Points:
- Three terms represent the composition of a compound – empirical formula, molecular formula and structural formula.
- Empirical formula is the minimum ratio of the elements present in a compound.
- Molecular formula is the exact number of the elements present in a compound.
- Structural formula is the representation of the exact nature of bonding of the elements present in a compound.
The nature of a chemical compound helps us in determining the reactions it undergoes, but, the crucial factor for this understanding is the knowledge of amount of the reactant. The amount of substance is quantified and this amount is represented in terms of “moles”.
IUPAC defines mole as:
“SI base unit for the amount of substance (symbol: mol). The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles.”
To understand the applicability of number of moles, we need to follow the definition of formula weight, molecular weight and molar mass. Simple addition of the atomic weights of elements in a formula is called the formula weight. If the formula is a molecular formula, then it is called the molecular weight and is sometimes called as molar mass if the atomic masses of the elements are added. Molecular weight is expressed in gmol-1 which means the weight of 1 mole of substance in grams is numerically equal to its molecular weight.
Key Points:
- The mole is the amount of substanceof a system which contains as many elementary entities (may be atoms, molecules, ions, electrons, other particles) as there are atoms in 0.012 kilogramof carbon-12.
- Molecular weight is expressed in gmol-1 which means the weight of 1 mole of substance in grams is numerically equal to its molecular weight.
Measurement is involved in every aspect of experimental chemistry and the most important part of this includes the understanding of the skill of the observer and the scope of measurement of a given instrument. To delve further into this, the two terms “accuracy” and “precision” need to be understood.
Let three students- A, B and C are doing a titration experiment in which the expected end point is reached at the burette reading of 23.5 ml. Each of the student repeats the experiment thrice and record their observations as follows:
| Observation No. | Burette reading (A) | Burette reading (B | Burette reading (C) |
| 1. | 24.6 ml | 23.5 ml | 23.5 ml |
| 2. | 24.6 ml | 22.1 ml | 23.5 ml |
| 3. | 24.8 ml | 28.7 ml | 23.4ml |
A has precision but no accuracy since the results are consistent but not close to the correct result.
B has reported one result close to the correct one, but the other two results are neither accurate nor precise.
C has reported the results which are both accurate and precise.
Error
This error resulting from the expertise (or lack of it) of the observer is called human error.
The second aspect of any measurement is the scope and limitation of an instrument. Let’s consider two graduated cylinders – X and Y each having a capacity of 10 ml. The cylinder X has 10 marks on it, each corresponding to 1 ml while Y has 100 marks on it, each corresponding to 0.1 ml. If we are to accurately measure a volume of 4.6 ml using any of these two cylinders, the cylinder Y will be the ideal one since it has markings that correspond to 0.1 ml. But, the cylinder X will only give a rough estimate since it has no markings between the those for 4 ml and 5 ml.
The error resulting from the limitation of any instrument is called the instrumental error.
Key Points:
- Any kind of measurement is a quantitative analysis.
- All measurements contain some degree of error.
- Human error results from the level of expertise of the observer.
- Instrumental error results from the limitation of the instrument.
Volumetric analysis is a practical approach towards accurate measurement of concentration, molecular mass, purity percentage, formula of compounds, percentage composition of an element and stoichiometry of a chemical equation. It involves 3 important techniques. The first one is the use of apparatus like burette, pipette and volumetric flasks. These are specially made to offer the highest degree of accuracy. The second aspect is the use of balance in weighing. Most balances come with a minimum and a maximum range – both in terms of weight measured and decimal places correctly reported. The third aspect is the use of appropriate indicators. Most of the titrations require external indicators for detecting the end points which essentially means the change in pH of the system.
Some of the common examples of indicators are:
- Phenolphthalein for strong acid vs strong base reactions.
- Methyl orange for strong acid vs weak base reactions.
- Starch for titrations involving iodine and thiosulfate.
- Potassium chromate and fluorescein for Silver nitrate titrations.
- Volumetric analysis can further be classified into 3 techniques depending on the nature of reactions:
- Acid-Base titrations which involve the reaction of an acid and a base.
- Redox titrations which includes redox reaction between the analyte and titrant as the key reaction.
- Complexometric titration which involves the formation of a colored complex compound.
Key Points:
- Volumetric analysis depends on the use of accurate apparatus like burette, pipette and volumetric flasks.
- Accurate weighing of substances is the key to accurate results.
- Indicators are often required for establishing the end-point in a volumetric analysis.
- Acid-Base titrations, Redox titrations and Complexometric titrations are the major techniques in volumetric analysis.
Frequently Asked Questions
How is the amount of substance expressed?
The amount of substance is expressed in moles.
How do you define mole?
Mole is the amount of substance containing 6.02 x 1023 particles.
What is the unit of molecular weight?
The molecular weight of a substance is expressed in grams per mole (g/mol).
What is the importance of mole?
Mole provides a universal system to calculate the amount of substances and makes it easy to deal with them in chemical labs.
References:
- University of Waterloo: http://www.science.uwaterloo.ca/~cchieh/cact/c120/formula.html
- http://www.knockhardy.org.uk/sci_htm_files/15volan.pdf
- Modern Abc Chemistry by Dr. S.P. Jauhar





