Table of Contents
Key Information & Summary
- Amino acids are building blocks of proteins.
- Based on the chemical nature of R group, amino acids are categorized as non-polar, polar, positively and negatively charged amino acids.
- Amino acids exist as zwitterions and are optically active.
- Proteins are important biomolecules made up of amino acids linked via peptide bonds.
- Proteins have four levels of structural organization including primary, secondary, tertiary and quaternary structure.
- Deoxyribonucleic acid (DNA) is a double helix made up of nucleotides and contains genetic information.
- Structure of DNA includes two strands that are held together by hydrogen bonds between nitrogenous bases in the nucleotides.
- In cells, DNA is present in nucleus, mitochondria and chloroplast and it replicates through semiconservative replication.

AMINO ACIDS
Amino acids are the building blocks of proteins. Around 700 amino acids have been discovered in nature. However, there are 20 important amino acids that are present in proteins.
Types of amino acids
An amino acid contains an alpha carbon attached to an amino group, a carboxyl group, hydrogen atom and R group. Based on the nature of R group, amino acids are classified as non-polar, polar, positively charged and negatively charged amino acids. Amino acids are named with a three letter code, e.g. glycine as Gly and also with one letter code e.g. glycine as G.
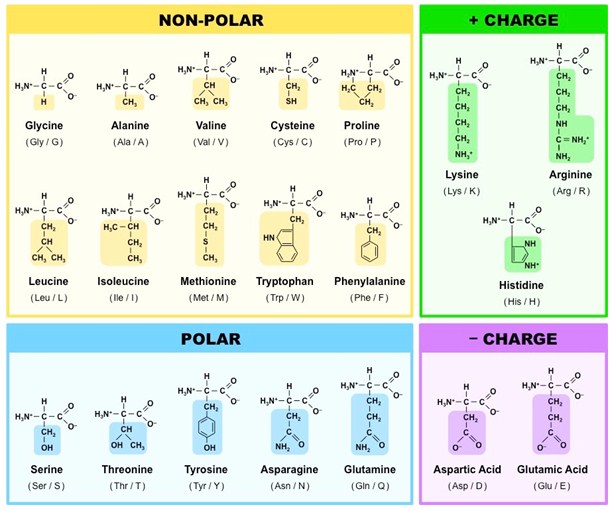
Zwitterions
The amine group in amino acids is basic while the carboxylic group is acidic. At neutral pH, the –NH2 group picks up proton from –COOH group so that NH3+ is positively charged while COO- is negatively charge. Overall charge of the entire molecule is zero. This type of molecules that have one positively charged and one negatively charged functional group such that the overall charge of the molecule is zero are called zwitterions. Amino acids with an uncharged R group exist as cations and anions at low and high pH, respectively.
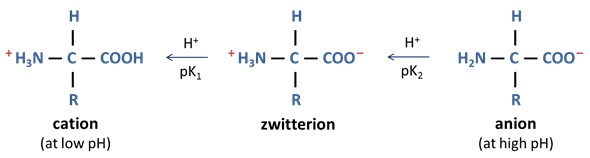
The point along pH scale where overall charge on an amino acid is zero is called isoelectric point (pI). Amino acids have different pI based on their R groups.
Read more about Organic Synthesis
Optical activity of amino acids
The alpha carbon in amino acids is chiral centre that is attached to four different groups (except glycine in which chiral carbon is attached to two H atoms). Therefore, amino acids are optically active molecules and may exist as L and D amino acids. These pairs of amino acids that are mirror images of each other are called enantiomers. Naturally occurring amino acids are L amino acids.
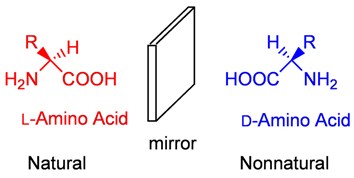
Proteins
Proteins are the most diverse biomolecules and play a significant role in various biological processes. Enzymes are the most important functional proteins that catalyse biological reactions. Proteins are sensitive to heat and pH changes. They often undergo irreversible changes in structure, called protein denaturation, due to extreme change in temperature or pH. Amino acids are the monomers of complex proteins.
Peptide bonds and polypeptides
Carboxyl group of one amino acid reacts with amino group of another amino acid to form a dipeptide linked through a peptide bond. When 100 or more amino acids link through peptide bonds, the resulting chain of amino acids is called polypeptide. One or more polypeptide chains constitute a protein.
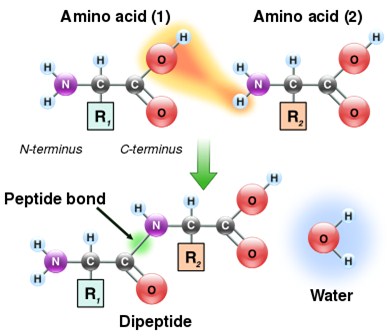
Structure of proteins
Proteins have four levels of structural organization including primary, secondary, tertiary and quaternary structures. Chain of amino acids joined by peptide bond forming a polypeptide is known as the primary structure of protein. The polypeptide chains may further bend or fold to form the secondary structure. There are two types of secondary structures including α-helix and β-sheets. The α-helix is a chain of amino acids that coil into a helix shape and stabilized by the formation hydrogen bonds between C=O and NH groups of various amino acids. The β-sheets are formed when the polypeptide chains arrange side by side and held together by hydrogen bonds between C=O and NH groups.
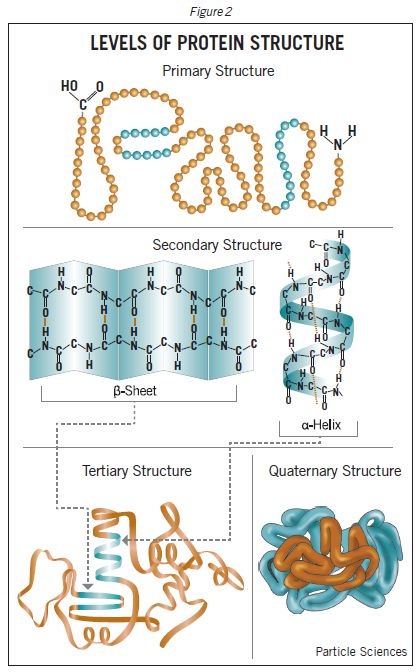
The side chain R groups of amino acids in polypeptide chain interact to through various interatomic forces like hydrogen bonds, hydrophobic bonds, disulphide bonds or van der Waals forces. This level of organization is known is tertiary structure. Two or more polypeptide chains may interact through hydrogen bonds, covalent bonds and/or hydrophobic interactions to form the quaternary structure. This is the most stable structure of proteins and gives a final 3-dimensional shape to the proteins.
DEOXYRIBONUCLEIC ACID (DNA)
Deoxyribonucleic acid (DNA) is an organic chemical compound that codes for genetic information. It is found in many viruses and all prokaryotic and eukaryotic cells.
Structure of DNA
Structure of DNA was first discovered by James Watson and Francis Crick in 1953. They found that two antiparallel strands of DNA are wound around each other to form a double helix. Each strand of DNA is made up of chain of nucleotides and each nucleotide contains a deoxyribose sugar, a phosphate group and one of four nitrogenous bases. Nitrogenous bases are divided into two categories including purines (adenine and guanine) and pyrimidines (cytosine and thymine). Nitrogenous bases join the two strands of DNA through hydrogen bonds such that cytosine always forms three H-bonds with guanine and adenine always forms two H-bonds with thymine. Nucleotides are joined together through covalent bonds between the phosphate groups of each nucleotide.
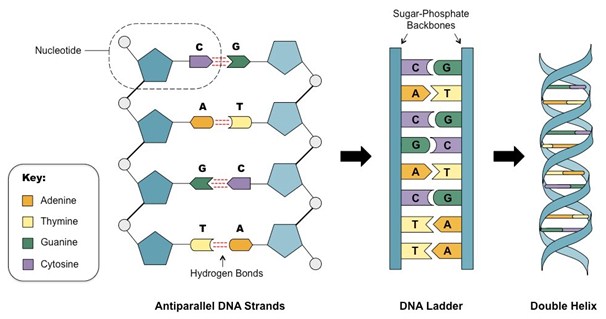
Cellular location and replication
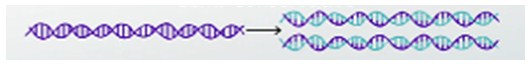
DNA is a stable and self-replicating molecule. Within a cell, DNA is wound around histone proteins and form compact structures called chromosomes. In eukaryotic cells, chromosomes are present inside the nucleus. DNA is also present in other eukaryotic cellular organelles like mitochondria and chloroplasts. During DNA replication, the two strands open up and each of the two strands guides the synthesis of a new strand. This method of replication is called semiconservative replication because the resulting DNA strand is composed of one old strand and one new strand.
DNA sequence
The order of nucleotides in DNA strand forms a “sequence”. A DNA sequence can be read like the sequence of letters in a book. This DNA sequence is transcribed into RNA during the process of “transcription” and resulting RNA sequence is “translated” into proteins. This is how genetic information in the DNA is converted to functional proteins.
Graphical Summary
Frequently Asked Questions
What are amino acids?
Amino acids are the organic molecules containing amino and carboxylic groups attached to the alpha carbon atom. They join to form long polypeptide chains and proteins.
What are Zwitter ions?
The amine group of amino acids is basic, while the carboxylic group is acidic. At physiological PH, the carboxylic group of amino acids loses its proton, so it exists as COO- while the NH2 group accepts H+ and exists as NH3+, but the overall molecule is neutral. This molecular structure is called the Zwitter ion.
How is a peptide bond formed?
A peptide bond is formed between two amino acids when the amino group of one amino acid reacts with the carboxylic group of others to form a chemical bond by releasing a water molecule.
What is the relation between amino acids, proteins and DNA?
Body proteins are synthesized according to the instructions encoded in DNA. The nucleotide sequence of DNA controls the amino acid sequence of the proteins.
References for further reading
https://www.youtube.com/watch?v=q4uBQFzPVhg
https://dlc.dcccd.edu/biology1-3/proteins
https://www.nature.com/scitable/topicpage/discovery-of-dna-structure-and-function-watson-397
If you like what you read, and you're teaching or studying A-Level Biology, check out our other site! We also offer revision and teaching resources for Geography, Computer Science, and History.





