Table of Contents
Key Information & Summary
- Key Points
- Equilibrium Constant ( k c )
- Reversible Reaction
- Dynamic Equilibrium
- Le Chatlier’s Principle
- Factors affecting Equilibrium
- Law of Mass Action
- Equilibrium constant of Pressure ( k p )
- Types of Equilibrium constants
- Important Processes/ Reactions
- Bibliography
Key points to remember:
- K c and k p are only affected by the temperature and no others factors which usually affect the equilibrium of a reaction.
- Higher value of the K c and k p indicates that the reaction is moving in the forward direction and vice versa.
- In expressions of K c , the reactants ( R ) and products ( P ) which are in solid state and are denoted by the solid state symbol ( s ), won’t appear as their concentration cannot be calculated.
- K c value of the reverse/ backward reaction is a reciprocal of the forward reaction’s value.
- In k p expression, the reactants ( R ) and products ( P ) with only gases state symbol ( g ) appear.
Equilibrium Constant ‘K c’ is defined as the quantity based on the concentrations of all the different reactions species at equilibrium in a reversible reaction.
Now, what is a reversible reaction?
Reversible reactions are chemical reactions which can proceed in both forward direction as well as in reverse direction at the same time. This type of reaction is denoted by a specific sign as mentioned in the example below between the reactant A and product B.
A ⇌ B
All reactions are bound to achieve a state at which both the forward reaction and the reverse reaction is taking place at the same rate. This state is known as the equilibrium and as the rates of forward and reverse/ backward reaction are equal and same thus the concentration of the reactants and products are constant at equilibrium. This is also known a Dynamic Equilibrium.
Now, what exactly is Dynamic Equilibrium?
Dynamic Equilibrium:
Rate of forward reaction = Rate of reverse/ backward reaction
“A stage in reversible reaction where rate of forward reaction becomes equal to the rate of reverse reaction.”
According to the “Le Chatelier’s Principle”:
“If an equilibrium of a reversible reaction is disturbed by some external factor, the reaction will shift in either direction (that is forward and reverse) to minimize the effect of the change that is to store the equilibrium.”
Now, what are the factors which can affect the equilibrium in a reaction?
Factors affecting Reversible reaction (Equilibrium)
-
Concentration:
by increasing the concentration of the reactants, the reaction moves in the forward direction and vice versa. The equilibrium will be stored at a new position.
-
Pressure:
(ONLY FOR GASES) by increasing the pressure, the volume will decrease, and the reaction will move in the direction where less moles of gases are present and vice versa.
-
Temperature:
By increasing the temperature of a reversible endothermic reaction (the reaction process which requires heat), it moves in the forward direction and vice versa.
-
Catalyst:
In a reversible reaction, rate of forward reaction is equal to the rate of reverse/ backward reaction. The catalyst does not affect the yield that is the amount of the product but only helps in attaining the equilibrium in less time because it increases both the rate of forward reaction and rate of reverse/ backward reaction.
According to the law of mass action:
“the rate of a reaction is proportional to active masses (concentration) of the reactants.”
Note: the concentration is shown by square brackets [ ]. And the unit of concentration is mol.dm-3
aA + bB ↔ cC + dD
rate of forward reaction α [ A ] a [ B ] b
rate of reverse/ backward reaction α [ C ] c [ D ] d
OR
rate of forward reaction = k f [ A ] a [ B ] b
rate of reverse/ backward reaction = k r [ C ] c [ D ] d
at equilibrium:
rate of forward reaction = rate of reverse reaction
k f [ A ] a [ B ] b = k r [ C ] c [ D ] d
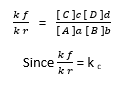
K f = rate constant of the forward reaction
K r = rate constant of the reverse/ backward reaction
K c = equilibrium constant of the concentration
Therefore,
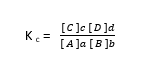
Question: write K c expression for the following reaction.
2SO 2 (g)+ O 2 (g)⇋ 2SO 3 (g)
Answer:

Expressions for k p (equilibrium constant for pressures):
If in a reversible reaction, gases are present, k p expressions can also be written in addition to k c
Types of equilibrium constants for different types of reactions
- Gas-phase reactions which use units of partial pressure ( atm ) : K p
- Dissociation of water: dissociation constant of water, K w
- Dissociation of acids: acid dissociation constant, K a
- Reaction of bases with water: base hydrolysis constant, K b
- Solubility of precipitates: solubility product, K s p
- Formation of complexes: formation constant, K f
Important reactions in which equilibria plays a significant role
-
Haber’s process
Manufacture of ammonia
N 2 + 3 H 2 ↔ 2 NH 3 ( ∆ H = negative )
Conditions:
- Pressure = 200 atm
- Temperature = 350 – 450 degree Celsius
- Catalyst = iron or iron ( III ) oxide
-
Contact process
Manufacture of Sulphuric acid
( ∆ H = negative )
- S ( s ) + O 2 → S O 2 ( g )
- 2 S O 2 + O 2 ↔ 2 S O 3
- S O 3 ( g ) + H 2 S O 4 → H 2 S 2 O 7 ( l )
- H 2 S 2 O 7 ( l ) + H 2 O → 2 H 2 S O 4
Conditions:
- Pressure = 1 – 2 atm
- Temperature = 350 -450 degree Celsius
- Catalyst = vanadium ( V ) oxide ( V 2 O 5 )
BIBLIOGRAPHY:
- https://www.khanacademy.org/science/chemistry/chemical-equilibrium/equilibrium-constant/a/the-equilibrium-constant-k
- https://www.chemicool.com/definition/equilibrium_constant.html
- https://www.tissuegroup.chem.vt.edu/chem-ed/equilibr/equil-constant.html
- http://www2.hkedcity.net/sch_files/a/l11/l11-chm/public_html/page32.html





