Table of Contents
Key Information & Overview:
- Definitions
- Hydrolysis of Proteins
- Structure of the Electrophoresis apparatus
- The Science behind Electrophoresis
- Reasons for the Changes in Speed
Definitions
Electrophoresis is a very common test run in labs which are used to identify and differentiate between different forms of protein. This process not only finds out which state the protein is in, but it also purifies it. This test can be used to find out the different types of amino acids that are formed in the hydrolysis of proteins.
Isoelectric Point is the pH value of the amino acids when they are added in water. This is because of the dissociation of the H+ ion from the amino acids.
Electropherograms are lines that are formed from the movement of the proteins to the electrodes.
Hydrolysis of Proteins
Hydrolysis of proteins is the breaking down of the peptide links (which makes up the protein) into amino acids. There are two ways in which the hydrolysis of the peptide links can be done:
- Reacting proteins with 6M of hydrochloric acid, HCl, for 24 hours
- Reacting it with an enzyme for several hours
The first method is slow and old and the second method is new and fast.
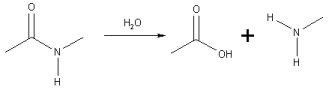
(The peptide bond is broken in the hydrolysis of peptides (protein) into two amino acids. The -OH gets attached to the C=O and the -H gets attached -N)
The longer the chain of the peptide, the longer the hydrolysis process will take place. This is because more time is taken to break the peptide bond and other bonds like Van der Wall forces of attraction between the atoms.
Structure of the Electrophoresis apparatus
In short, electrophoresis is a biochemical analytical technique which can be used as a test to find out if the protein is a peptide or an amino acid. Even though electrophoresis can be used for peptides, polypeptides, amino acids, and even different proteins as well, for the sake of clarity, we will only discuss amino acids. However, the same concepts can be applied to all types of molecules mentioned above.
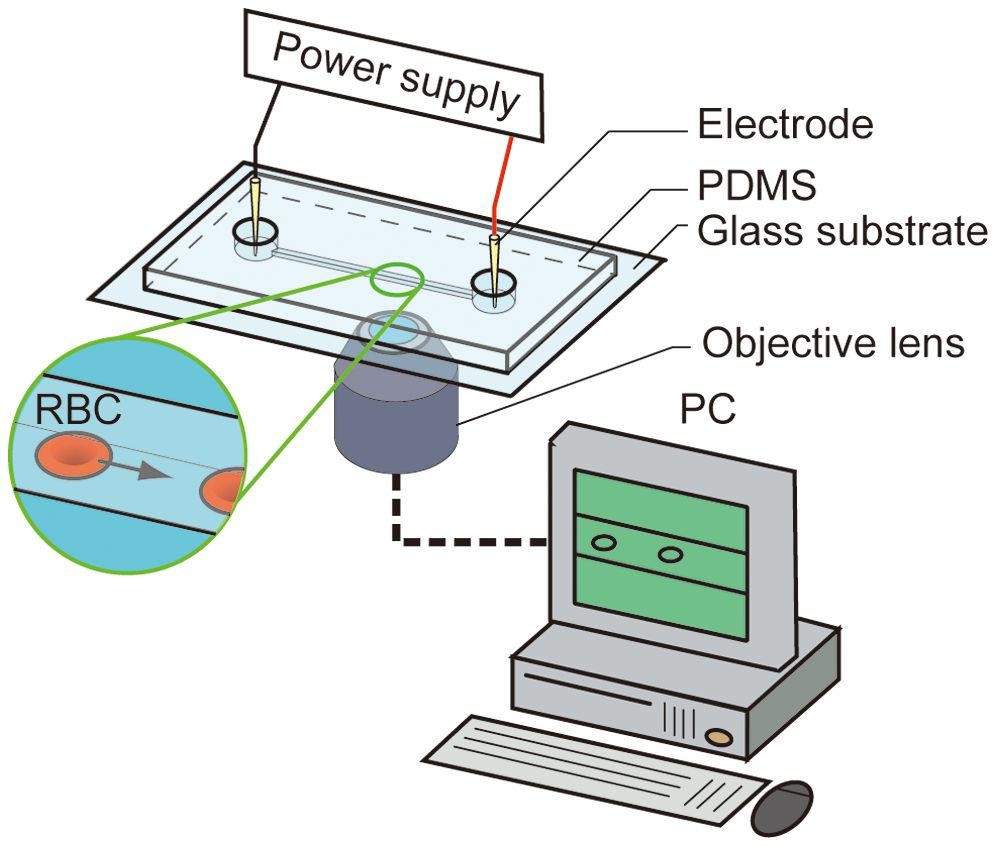
(A simple set up of an electrpherosis machine. In this picture, the Red Blood cell is being examined and tested for peptides and amino acids.)
The electrophoresis machine has two electrodes: a positively charged electrode and a negatively charged electrode. When the sample that has to be examined is placed between the negatively and positively charged electrodes, the positive ions will be attracted and will move towards the negatively charged electrode and the negative ions will be attracted and move towards the negatively charged electrode.
The Science behind Electrophoresis
Positively charged amino acids and negatively charged amino acids are formed due to amino acids being amphoteric. That means that they have both: an acidic nature and an alkaline nature. An isoelectric point (IEP) is the pH value when the amino acid is dissolved in water. When H+ ions are added in the solution, the pH value decreases and when H+ ions are subtracted from the solution, the pH value decreases. The Isoelectric Points of different amino acids is different.
The sample of amino acids is placed in a buffer solution. A buffer solution resists the change in the pH of the solution. The buffer solution is kept at the Isoelectric Point of one known amino acid. The amino acids with Isoelectric Points higher than the pH of the buffer solution become positively charged. The amino acids with Isoelectric Points lower than the pH of the buffer solution become negatively charged. The amino acids with the Isoelectric point that is equal to the pH of the buffer solution remain uncharged.
When the charge is applied to the electrodes, the different amino acid will move at different speeds to the respective electrode. The speed at which they move and to which electrode they travel to is recorded and compared on the glass substrate. Series of bands are formed from their movements which are called Electropherograms.
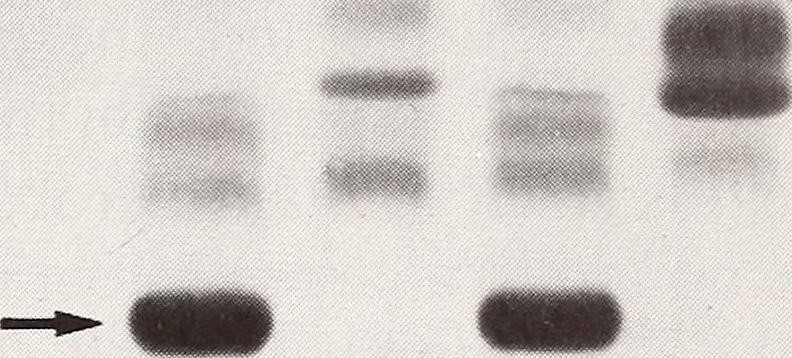
(A strong colour on the electropherogram means that the amino acid is moving at a faster rate. A weaker colour indicates that the amino acid is moving at a sower rate.)
Reasons for the Changes in Speed
The speed at which the amino acid moves to its respective charged electrode varies because of different reasons:
- The charge on the amino acids
Amino acids that have a bigger charge will move at a faster rate compared to the amino acids that have a smaller charge. The charge is not due to the amino acid itself but due to the charge on the side chain.
- The shape of the amino acids
Spherical amino acids move at a faster rate than irregularly sized amino acids.
- The size of the amino acids
Amino acids that have smaller alkyl chains attached to them move at a faster rate than amino acids that have a bigger alkyl chain attached to them.
- The temperature of the system
If the temperature of the system is high, the kinetic energy of the amino acids increases and thus the amino acids travel to the charged electrode at a faster rate.
- The size of the pores in the gel
If the pores of the gel are large, the amino acids will move at a faster rate to the electrodes compared to in a gel which has smaller pores.
Summary
- Electrophoresis is a process of distinguishing between different types of proteins, peptides and amino acids
- Electrophoresis can be used to find out what type of amino acids are produced in the hydrolysis of proteins
- The apparatus has positively and negatively charged electrodes.
- Proteins, peptides and amino acids can be cations and anions. The cations get attracted towards the positive electrode and the anions get attracted towards the negative electrode.
- Electropherograms can help compare the different types of proteins, peptides and amino acids.
- The speed of the movement of proteins, peptides, and amino acids can differ due to the size of the charge, size of the molecule, shape of the molecule and the temperature of the system.





