Table of Contents
Key Information & Overview:
- Definitions
- How does Electronegativity occur?
- Values and Trends of the Electronegativities of Atoms
- Electronegativity in Covalent Bonds
- Electronegativity in Ionic Bonds
- Bond Polarity
Definitions
Electronegativity of an atom is its ability to attract the electrons towards itself.
Bond polarity is the unequal distribution of electrons in a covalent bond.
The dipole shows to which atom the electrons are more attracted to.
The nuclear pull is the attraction of the negatively charged electrons towards the nucleus because of the presence of positively charged protons in it.
How does Electronegativity occur?
Some atoms have a nuclear pull that is stronger than others. This is because of two reasons: the atomic radius and the number of protons in the nucleus. The smaller the radius, the closer the electrons are. This puts them in the range of the nuclear pull and gets attracted towards the nucleus. A higher number of protons means the strength of the nuclear pull increases and the electrons have a stronger attraction.
Values and Trends of the Electronegativities of Atoms
The values of the electronegativity of atoms can be calculated using complicated formulas but that is not in the syllabus. But, it is better to go over through them and have an idea.
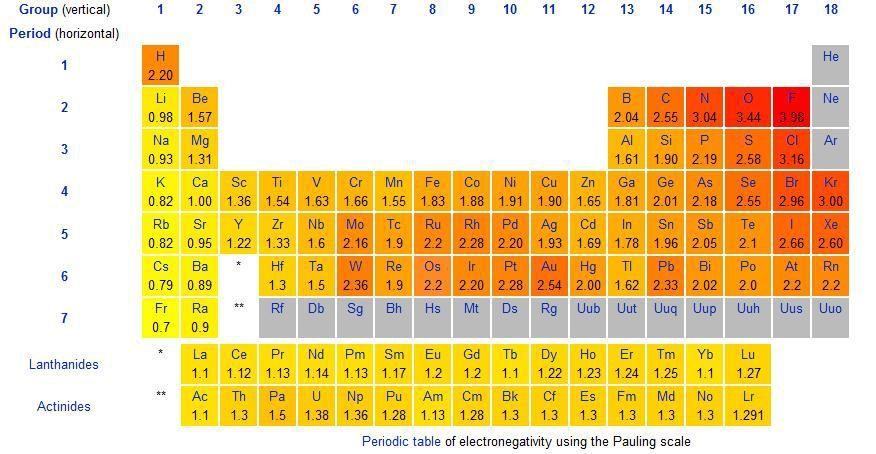
(This periodic table shows the elements and their corresponding values)
The electronegativity of different atoms can be calculated and have values assigned to it but the general rule of thumb is that the electronegativity of atoms increases along the period and decreases down the group. This is because the atomic radius decreases along the period but the number of protons increases. The nuclear pull increases and the electrons get attracted towards the nucleus. Down the group, the radius of the atom increases and the electrons are far away and the nucleus strength is relatively weaker on the electrons that are further away from the nucleus. In the 2nd period, Fluorine is the most electronegative with the value of 3.98 and Lithium is the least electronegative with the value of 0.93. This supports the explanation given above as both Fluorine and Lithium are on the two extremes of the 2nd period.
Electronegativity in Covalent Bonds
In a covalent bond, the electron pair/s is shared between two atoms, making it stable. If the two atoms bonded have a similar electronegativity value,the bonded electron stays in the middle and both the atoms have an equal sharing. For example, if two Fluorine atoms are bonded, the bonded electron will stay in the middle.
However, if the electronegativity of the two atoms is not similar, the electrons that bonded will get pulled towards the atom with the higher electronegativity. This will lead to an unequal distribution of electrons and causes a dipole to be formed. A dipole points towards the more electronegative atom and shows where the electrons are being attracted to more and moving towards which atom.
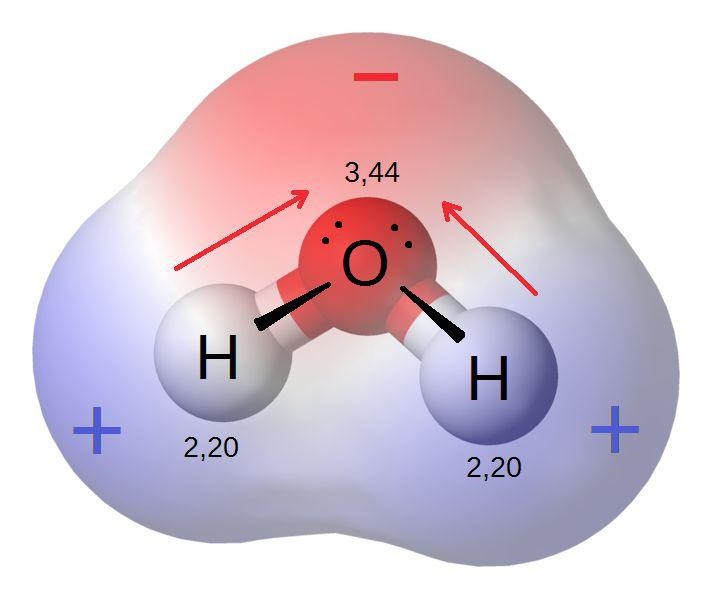
(This is H2O which is a covalently bonded molecule. Oxygen is more electronegative than Hydrogen because of it being further along the period than Hydrogen. The electrons move towards the more electronegative atom which is the Oxygen and dipoles are formed from Hydrogen to Oxygen showing the movement of electrons.)
As the electrons move towards the more electronegative atom, a time comes when the more electronegative atom attracts a large concentration of negative electrons. The concentration of electrons develops a partially negative charge over the more electronegative molecule. The less electronegative molecule has an electron depletion and thus develops a partial negative charge. The partial negative charge is represented with -δ. The partial positive charge is represented by +δ. The atom towards which the dipole points to becomes partially negative and the other atom becomes partially positive.
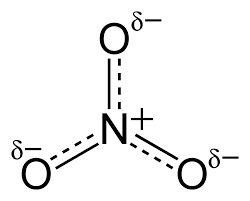
(This is NO3 which is a covalently bonded molecule. Oxygen is more electronegative than Nitrogen as it is further along the period. The Oxygens, in this case, develops a partial negative charge because they attract the electrons towards themselves and become concentrated with electrons. Nitrogen becomes electron deficient develops a partial positive charge.)
The electronegative difference is the reason why covalently bonded molecules can react. The partial positive and partial negative atoms act as positively and negatively charged atoms respectively and react.
Electronegativity in Ionic Bonds
Ionic bonds are formed when a metal and a nonmetal react to form a compound. Metals are on the right side of the periodic table and have lower electronegative values. The nonmetals are on the left side and have high electronegativity values. This causes the electronegativity difference to be very high. Because of the high difference, instead of sharing the electrons, the nonmetal pulls the electron towards itself and makes it part of its valence electron and an ionic bond is formed. The nonmetal becomes negatively charged and the metal becomes positively charged. The metal becomes positively charged because it loses electrons.
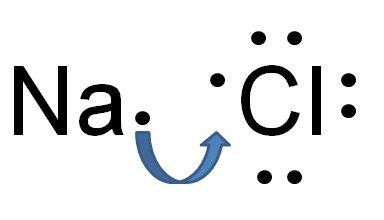
(This is NaCl which has an ionic bond. Chlorine is more electronegative than Sodium. Thus, Chlorine attracts the electrons towards itself. The electronegativity difference is big and instead of sharing the electron, Chlorine takes Sodium’s electron and becomes Cl-1. Sodium now has one less electron and becomes Na+1. The positive ion and the negative ion are attracted to each other and they form an ionic bond.)
Bond Polarity
When many atoms are covalently bonded to form one molecule, there can be many dipoles. If the dipoles are of the same length and are pointing away from each other, they cancel out each others' effect and the bond is not polar. This means that the molecule is not reactive. When the dipole sum is zero, there is no bond polarity Equal bond lengths are of the bonds with the same electronegative difference.
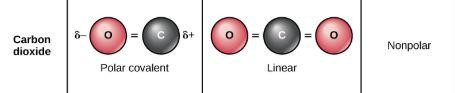
(C = O bond produces a dipole but the dipoles cancel each other out and the dipole sum is 0. CO2 is nonpolar.)
There can be exceptional cases in which non-polar molecules react. We can take an example of N which is in the air. It is nonpolar and does not react but when struck with lightning, it
produces nitrogen oxides.
Some dipoles do not cancel out and they make a dipole sum which is more than zero. This means the bond is polar and can react. Polar molecules have a higher reactivity.
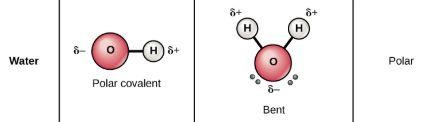
(Because the O-H bonds are at an angle, they do not cancel each other out. The dipole sum is nonzero and H2O is polar.)
Some bonds do not produce a dipole even with the electronegativity difference. In our course, we only need to remember that C-H and S-H bonds do not produce a dipole.
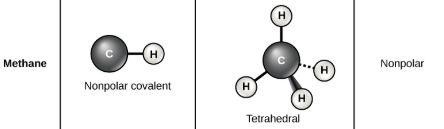
(C-H bond does not produce a dipole. Thus, CH4 is nonpolar.)
Summary:
- Electronegativity is the ability to attract electrons.
- Electronegativity increases along the period and down the group.
- In covalent bonds, electronegativity difference causes dipoles to be formed. Dipoles cause partial positive and partial negative ends to be formed
- In ionic bonds, electronegativity difference causes positive and negative ions to be formed
- If the dipole sum is zero, the bond is nonpolar. If the dipole sum exists, the bond is polar.
- C-H and S-H bonds do not produce dipoles.





