Table of Contents
Key Information & Summary
- Crystals are solids in which all of the atoms are arranged in repeating patterns
- There are 4 types of crystals: metallic, ionic, covalent, and molecular
- Metallic crystals have metallic bonds
- Ionic crystals have ionic bonds
- Covalent crystals have covalent bonds
- Molecular crystals are held together through intermolecular forces, such as hydrogen bonds
What are crystals?
Crystals are solids in which all of the atoms are arranged in a repeating pattern. These highly-ordered atoms form a structure known as a crystal lattice which extends in all 3D directions. There are four types of crystal structures:
- Metallic crystals
- Ionic crystals
- Covalent crystals
- Molecular crystals
Crystals are formed through a process called crystallisation.
Metallic crystals
Metallic crystals are structures made entirely of metal elements. The atoms in these structures are bonded through metallic bonding – this is a type of bonding in which an electrostatic force between a cloud of delocalised electrons is attracted to the positively-charged metal atoms. This structure gives metallic crystals a number of properties:
- They have a very high melting point and a very high boiling point
- They conduct both electric currents and thermal energy (heat)
- They are ductile – this essentially means that they are capable of being ‘stretched out’ to form thin wires
- They are malleable – this means that they are capable of being ‘hammered out’ to form thin sheets without breaking
Metallic crystals are usually found in one of the following three arrangements:
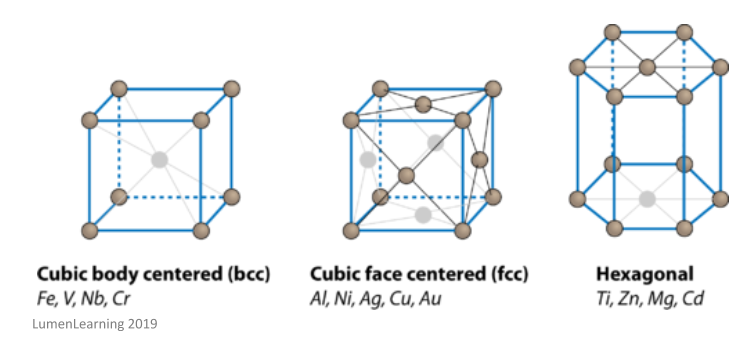
The atoms in these structures are packed very closely together, as this is the most efficient arrangement and helps to make the gaps between each atom as small as possible.
These crystals are easily identified through their shiny surfaces. This is because the delocalised electrons are not tied to any one specific bond, and so can move freely throughout the crystal lattice, absorbing and emitting light of any wavelength.
Ionic crystals
Ionic crystals are crystal structures in which the atoms are held together through ionic bonds. There are no free electrons in this structure, and rather the atoms are bound through electrostatic force arising from a difference in charge.
The image below shows a good example of an ionic crystal: common table salt (also known as NaCl). Here you can easily see the ions with opposite charges in the lattice structure.
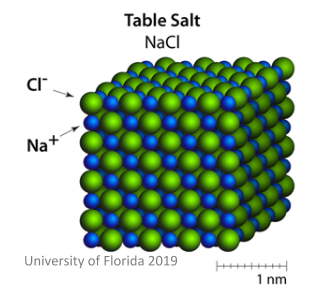
There are a number of properties which arise from this structural arrangement:
- They do not conduct electricity when in the solid form – this is because the ions in this structure are in a fixed position and cannot be moved (when they are dissolved in water, the ions dissociate and can them carry the electrical charge)
- They have very high melting points – this is because the ionic bonds in the lattice are very strong and require a lot of energy to break them
- They are very hard and brittle – this is because, when under force, ions with the same charge are forced to be close to one another and cause huge electrostatic repulsion
How stable an ionic crystal in can be measured in terms of something called lattice energy. The higher the lattice energy of an ionic crystal, the more stable the structure is. It is defined as the following:
Lattice energy is the amount of energy released when one mole of an ionic crystal is formed from one mole of positive gaseous ions and one mole of negative gaseous ions (when these are separated by an infinite distance).
Covalent crystals
Covalent crystals are structures in which the atoms are bonded through covalent bonds – this is the type on bond where the atoms share their outer electrons. You need to know that these covalent bonds are directional, which essentially forms one huge interlocking crystal structure.
This bonding gives covalent crystals a number of properties:
- They have very high melting points and very high boiling points – this is because the covalent bonds in the lattice are incredibly strong an require a large amount of energy to break
- They don’t conduct electricity – this is because there are no free electrons in the lattice, and so no charge carriers (graphite is the only exception to this rule, which can conduct electricity)
The image below shows the structure of diamond, a covalent crystal. The red lines in the image represent these very strong covalent bonds. The blue atoms are representative of carbon atoms which sit on ‘lattice points’, and the green atoms show atoms which are not lattice points.
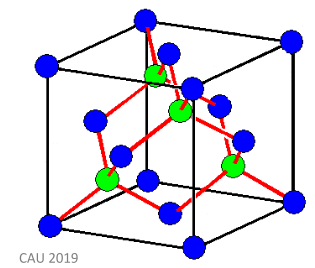
Molecular crystals
Molecular crystals are structures in which the atoms are bonded through weak intermolecular forces. If the structure is formed of nonpolar crystals, then the forces will be dispersion forces. If the structure is formed of polar crystals, then the forces with be dipole-dipole. In structures, such as ice (shown in the image below), the forces will be hydrogen bonds.
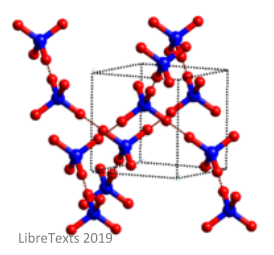
These bonds give molecular crystals a number of properties:
- They have low melting and boiling points – this is because the intermolecular bonds between the atoms are fairly weak and take little energy to break
- They are poor at conducting electricity – this is because there are no free electrons to act as charge carriers throughout the lattice
The intermolecular forces holding these molecular crystals together are a lot weaker than covalent bonds or ionic bonds.
References and further readings:
[1]. https://study.com/academy/lesson/crystal-definition-types-structure-properties.html
[2]. https://courses.lumenlearning.com/cheminter/chapter/crystal-structures-of-metals/
[3]. https://www.tf.uni-kiel.de/matwis/amat/def_en/kap_2/basics/b2_1_6.html
[4]. https://courses.lumenlearning.com/introchem/chapter/covalent-crystals/
[5]. http://web.fscj.edu/Milczanowski/psc/lect/Ch10/slide7.htm





