Table of Contents
Key Information & Summary
- A chemical species is a set of molecular entities which are chemically identical and can explore the same molecular energy levels at a set timescale
- If two conformational isomers interconvert slowly enough to each be detected by different NMR spectra, they would be defined as two different chemical species
- In supramolecular chemistry, supramolecular structures whose interactions have been formed through the creation or breaking of certain intermolecular bonds are defined as the same chemical species
What does ‘chemical species’ mean?
Put simply, if an atom is identical to another atom, they are defined as being the same chemical species. This is also true of molecules, in that if one molecule is identical to another, they are the same chemical species.
A chemical species can also be defined as a set of molecular entities which are chemically identical and can explore the same molecular energy levels on a set timescale.
For example, a bottle full of water contains molecules of exactly the same chemical species. Moreover, a bar of solid gold contains atoms of the exact same chemical species.
The term ‘chemical species’ can also refer to the form a chemical exists in when it is found in a solution. For example, when NaCl is dissolved in solution, you don’t actually find NaCl. Rather, you have Na+ ions and Cl- ions – this is because the NaCl has dissociated. As such, the chemical species in this solution will be the Na+ ions and the Cl- ions. This rule holds true for any strong electrolytes. They are said to have ionic species in solution.
For electrolytes which do not dissociate in solution, the chemical species is defined as being the same as it was before it was added to the solution. They are said to have molecular species in solution.
Weak electrolytes are known to have both ionic and molecular species in solution – this is because some molecules dissociate, and some don’t.
In addition to this, a group of molecules with different isotopes are also classed as being of the same chemical species.
Chemical species and NMR
NMR (nuclear magnetic resonance) spectroscopy is a chemical technique used to determine the structure of organic chemical compounds – it is also the only spectroscopic method which gives a complete analysis of the entire spectrum. It is a non-destructive technique and requires as little as a milligram of the analyte to produce good data.
NMR is based on the theory that all nuclei are electrically charged and possess spin. This means that if an external magnetic field is applied to the nuclei, an energy transfer is possible between the base energy level and a higher energy level. When this spin returns back to its base level, energy is emitted. The frequency at which this happens can be measured and then processed to give an NMR spectrum.
NMR can be used to identify conformational isomers. These are isomers that are produced through rotation of σ bonds – they also interconvert (switch between the two) very quickly at room temperature. One example of a molecule which does this is butane, which can be shown in the image below.
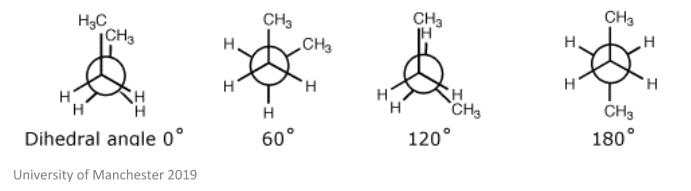
This theory becomes important when talking about chemical species. Due to the fact that these isomers can convert from one form to another, a mixture of two isomers may convert relatively slowly. As we have discussed before, chemical species are defined as such at a certain point in time. This means, if these two isomers interconvert slowly enough to each be detected by different NMR spectra, they would be defined as two different chemical species. This also goes to say that a mixture of the same isomer may be considered as the same chemical species, as the two are in equilibrium.
Chemical species in supramolecular chemistry
To first understand how chemical species are defined in supramolecular chemistry, you need to know the basics of the discipline. Supramolecular refers to the area of chemistry that deals with how molecules link up to form bigger ‘systems’ from a discrete number of subunits. The forces involved in supramolecular chemistry can range from weak forces (such as hydrogen bonding and electrostatic interactions) to stronger forces (such as covalent bonds). Studies in this area of chemistry focus primarily on the weaker and reversible non-covalent bonds between certain molecules, such as:
- Hydrogen bonds
- Hydrophobic forces
- van der Walls forces
- Metal coordination
- Pi-pi interactions
The study of these bonds is especially important in biological sciences, as they have proven to be important in molecular self-assembly and folding.
In terms of a chemical species in supramolecular chemistry, they are defined as supramolecular structures whose interactions have been formed through the creation or breaking of certain intermolecular bonds.
References and further readings:
[1]. https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/nmr/nmr1.htm
[2]. https://homepage.univie.ac.at/jeanluc.mieusset/Supramolecular%20Chemistry%201%20-%20Concepts.pdf





