Facts, Summary & Definition
- In redox reactions, one reactant is oxidised, and one is reduced
- The most common method of balancing redox reactions is the ion-electron method (which can also be called the half-reaction method)
- There are many steps in balancing a redox reaction, and all must be done in the correct order to give a correctly balanced redox reaction
What are redox reactions?
Redox reactions are a type of reaction in which one reactant is oxidised and one is reduced, hence the name. Both oxidation and reduction happen at the same time – in other words, simultaneously. An example of this is shown below.
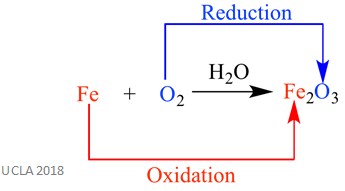
The above example of a redox reaction is the rusting of iron. As you can see, iron is being oxidised at the same time as oxygen is being reduced.
The most common way of balancing a redox reaction is the ion-electron method – this can also be called the half-reaction method. There are other ways, too, which will be shown here.
How to balance a redox reaction using the ion-electron method
In this method, the unbalanced equation is first converted into an ionic equation, then broken down into two half-reactions of oxidation and reduction. Each of these reactions is then balanced separately, then combined to give a fully balanced ionic reaction. The spectator ions are then added to the equation which converts it back into a molecular form.
It is important to follow these steps in the correct order.
- Write down the unbalanced equations
- Separate the redox reaction into half-reactions
- Assign oxidation numbers for each atom
- Identify and write out all of the redox couples in the reaction
- Combine these redox couples into two half-reactions
- Balance the atoms in each half-reaction
- Balance all other atoms except H and O
- Balance the oxygen atoms with H2O
- Balance the hydrogen atoms with H+
- If the reaction takes place in a basic medium, add one OH- to each side for every H+ present
- Balance the charges with e-
- Make the electron gain equivalent to electron loss in the half-reactions
- Add both of the half-reactions together
- Simplify the equation
You will need to check that all of the elements and charges are balanced when you have done all of these steps.
Example

You will need to practice the above method.
How to balance a redox reaction using the oxidation number method
The main principle in this method is that the gain in the oxidation number (otherwise known as the number of electrons) in one of the reactants must be equal to the loss in the oxidation number of the other reactant.
- Write the unbalanced equation
- Separate the process into half reactions
- Assign oxidation numbers for each atom
- Identify and write out all redox couples in the reaction
- Combine these redox couples into two half-reactions
- Balance the atoms in each half-reaction
- Balance all other atoms except H and O
- Balance the charge with protons or hydroxide ions
- Balance the oxygen atoms with water
- Make electron gain equivalent to electron loss in the half-reactions
- Add the half-reactions together
- Simplify the equations
You will need to check that all of the elements and charges are balanced.
How to balance a redox reaction using aggregate redox species (ARS) method
This is a more improvised version of the oxidation number method. However, this method can help to solve reactions that cannot normally be ‘cleanly’ separated into partial reactions.
- Write the unbalanced equation
- Identify redox couples in the equation
- Assign the oxidation numbers for each atom in the equation
- Identify and write out all redox couples
- Remove unnecessary redox couples
- Aggregate the redox species into one equation
- Balance redox atoms that aren’t part of a redox couples
- Balance non-redox atoms that are present only in redox species
- Combine the remaining reactions into two partial reactions
- Equalize the electron transfer in the two partial reactions
- Sum up the partial equations and determine the ‘free’ species
- Balance the charges and other atoms
- Add non-redox species and balance all atoms besides hydrogen and oxygen
- Balance the charges
- Balance the oxygen atoms
- Simplify the equation
You will need to check that all of the elements and charges are balanced.
For this method, it does not matter that the sum of the charges is, it just matters that it’s equal on both sides.
Further Reading
https://opentextbc.ca/introductorychemistry/chapter/balancing-redox-reactions-2/
https://www.periodni.com/balancing_redox_equations.php?eq=Ca%2BCl2%3DCaCl2
http://www.chem.ucla.edu/~harding/IGOC/R/redox_reaction.html





