Facts, Summary & Definition
- Atoms are the smallest unit of matter and combine to form molecules
- They contain protons, neutrons, and electrons
- More than 99% of an atom is empty space but electrons make atoms repel
- The number of protons in an atom determines its atomic number
- The number of protons and neutrons determines its atomic mass
What are atoms?
Atoms are the smallest unit of matter (something which can be physically touched) that retains all the chemical properties of an element. They combine to form molecules (which in turn form solids, gases, and liquids).
Atomic Particles
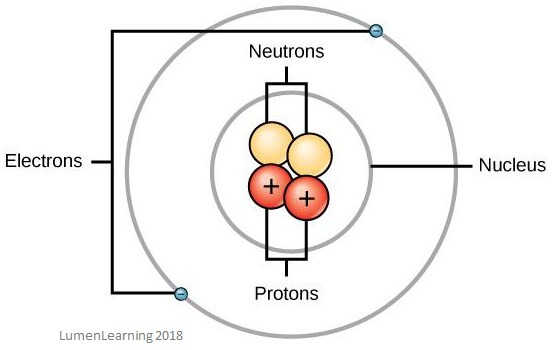
Atoms are made of three basic particles. These are electrons, protons, and neutrons – they are collectively referred to as subatomic particles.
The nucleus of the atom contains both protons (which are positively charged particles) and neutrons (which have no charge). These protons and neutrons are collectively called nucleons and are bound together by a force called the residual strong force. The number of neutrons relative to the number of protons helps to determine the stability of the nucleus.
Surrounding the nucleus are electron shells which contain the electrons (which are negatively charged particles. An atom’s properties depend on the number and arrangement of all their particles.
Electrons are the smallest of the subatomic particles (they are too small to be measured using techniques currently available to scientists) and have a negative charge. They are bound to the nucleus due to their opposite electrical charges. If an atom has either more or fewer electrons than its atomic number, then the atom becomes charged and is called an ion – if the atom has more electrons, the atom becomes more negative; if the atom has fewer electrons, the atom becomes more positive. Electrons have no internal structure and so they are called elementary particles.
Protons possess a positive charge and are 1836 times heavier than an electron. The number of protons in an atom is equal to the atomic number. Each proton is formed of two up quarks and one down quark – these are a type of elementary particle. Quarks are held together by a force called the strong interaction – this strong interaction is mediated by gluons (another type of elementary particle).
Neutrons are the heaviest subatomic particle and weigh 1839 times more than an electron. They possess no charge at all. Each neutron is formed of one up quark and two down quarks.
The image below shows the structure of an atom. You can see the nucleus and the electron shells:
Atomic Mass
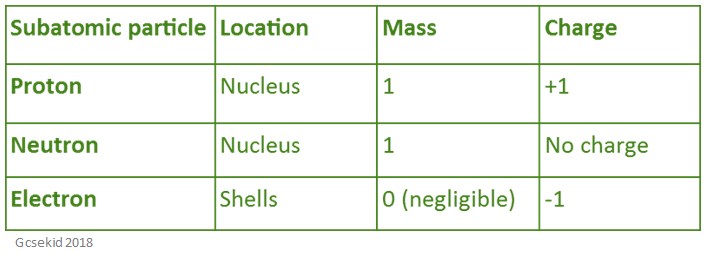
Protons and neutrons have the same mass of 1.67 × 10-24 grams – scientists usually define this amount of one Dalton. Whilst they are similar in mass, neutrons have no charge (whilst protons do). This means that neutrons will contribute heavily to an atom’s mass, but not its charge.
Electrons are much smaller and only weigh 9.11 × 10-28 grams – this means that they don’t significantly contribute to an atom’s mass but due to its charge.
The table below shows the location, mass, and charge of all of the particles that are in an atom.
Atomic Volume
More than 99% of an atom is empty space. However, the reason objects don’t just pass through one another is due to the electrons. The electrons that surround each atom are negatively charged, and so repel one another when bought near. This prevents atoms from occupying the same space as one another.
Atomic Number
The number of protons in an atom determines its atomic number – this is usually denoted by Z. This value will never change unless the nucleus somehow decays, or the nucleus is bombarded (nuclear physics). For example, carbon’s atomic number is 6 because it has 6 protons. The atomic number helps to distinguish elements from each other. The number of electrons in an atom is always equivalent to the number of protons – this explains why atoms have no overall charge.
Mass Number
The total number of protons and neutrons in an atom determines its mass number – this is usually denoted by A. Electrons are usually disregarded when calculating the mass of an electron as their mass is so negligible. You can calculate the number of neutrons in an element using the following equation:
mass number – number of protons = number of neutrons
Isotopes of the same element will have the same atomic number but a different mass number – this is because they can have a different number of neutrons. Most elements have many naturally-occurring isotopes. You also need to know that the atomic mass of an element is equivalent to the average of the relative abundance of all its isotopes. For example, carbon has three isotopes, but because carbon-12 makes up around 99% of all carbon – this is why carbon has the atomic mass of 12.
Further Reading
http://www.chem4kids.com/files/atom_structure.html
https://www.livescience.com/37206-atom-definition.html
http://www2.open.ac.uk/openlearn/periodictablephase2/atom.html





