Facts, Summary & Definition
- Anisotropy is the property of a substance to show variations in physical properties along different molecular axes
- These physical properties can be absorbance, electrical conductivity, and thermal conductivity etc.
- Anisotropic materials are the opposite of isotropic materials
What is anisotropy?
Anisotropy is defined as the property of a substance to show variations in physical properties along different molecular axes. It is usually observed in crystals and liquid crystals – it is less commonly seen in liquids. It can also be defined at the difference (when measured along different axes) in a material’s mechanical and physical properties – these include absorbance, conductivity, and tensile strength etc.
Crystal Chemistry
In order to understand anisotropy, you will need to know some basic crystal chemistry. A crystal is a solid material in which the constituents (e.g. atoms, molecules, and ions) are arranged in a repeating pattern which extends in all three dimensions. There are three main types of crystalline solids: ionic, metallic, and covalent.
Ionic Crystalline Solids
Ionic crystalline structures are formed of alternating cations (positively charged) and anions (negatively charged). This type of crystal are hard and brittle – they also have high melting points. As solids, they are not able to conduct electricity, but they do when molten or in an aqueous solution. An image of an ionic crystalline structure is shown below.
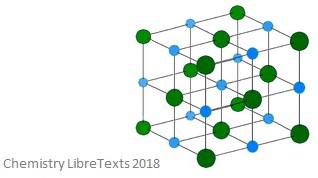
Metallic Crystalline Structures
Metallic crystalline structures are formed of metal cations (positively charged) surrounded by a so-called ‘sea’ of mobile valence electrons (these can also be called delocalized electrons, in which, they do not belong to any one specific atom, but are still able to move throughout the entire crystal structure). This means that metallic crystalline structures are excellent at conducting electricity. An image of a metallic crystalline structure is shown below.
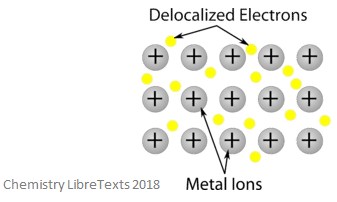
Covalent Crystalline Structures
Covalent crystalline structures are formed of atoms at the lattice points of the crystal – each atom is bonded covalently to its neighbouring atoms. This network is 3D and, much like the ionic structure, is very hard and brittle. They also have very high melting points and boiling points. These structures are made of atoms rather than ions, and so they do not conduct electricity. An image of a covalent crystalline structure is shown below.
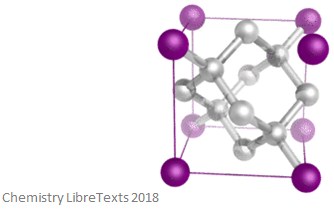
Anisotropy Explained
The image below shows a crystal lattice structure – you can assume in this case that all of the atoms are the same element.
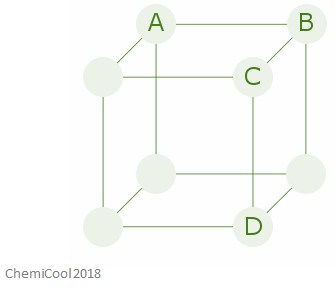
The distances A-B, A-C, and A-D are all different – this means that the physical and mechanical properties in this crystal will all be different across these different axes.
Anisotropic properties include:
- Hardness / cleavability
- Elasticity, expansion properties
- Electric / thermal conductivity
- Electric polarizability, magnetization
Anisotropic materials are the opposite of isotropic materials. These are material such as glass where no long-range order exists, and so the mechanical and physical properties are identical in all directions.
Further Reading
https://www.nde-ed.org/EducationResources/CommunityCollege/Materials/Structure/anisotropy.htm
https://chemistry.tutorvista.com/inorganic-chemistry/anisotropy.html





