Facts, Summary & Definition
- An amphoteric molecule is a molecule that can act and both a base and an acid
- All amphoteric molecules contain a hydrogen atom
- Water, amino acids, hydrogen carbonate ions, and hydrogen sulphate ions are all amphoteric
What is amphoterism?
An amphoteric molecule is a molecule that can act as both a base and an acid. It comes from the Greek word ‘amphoteroi’ which means ‘both’. The chemicals involved in the reaction dictate whether an amphoteric molecule will act as a base or acid.
Since all amphoteric compounds must be able to donate a proton, they all contain a hydrogen atom. Some examples are water and amino acids.
It applies to acids and bases according to the Brønsted-Lowry theory and Lewis theory.
Water as an amphoteric molecule
Water is one of the most well-known amphoteric molecules.
If a base, such as NH3, is involved in the reaction then water can act as an acid. This is because the water donates a proton to the base and is so converted to a hydroxide ion. The full equation for this is shown below:
H2O + NH3 → NH4+ + OH-
If an acid, such as HCL, is involved in the reaction then water can act as a base. This is because water will accept a proton from the acid and so is converted to a hydronium ion. The full equation for this is shown below:
H2O + HCl → Cl- + H3O+
Water as an amphoteric molecule is shown well in the image below.
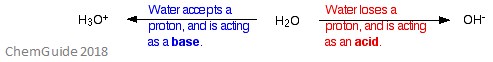
A water molecule can even act as an amphoteric molecule in a sample of pure water. Roughly 6 in 108 water molecules undergo the following reaction:
H2O(l)+H2O(l)→H3O+(aq)+OH−(aq)
This reaction is called autoionisation. It occurs in every solution, no matter if the sample of pure or it is part of a solution.
Amino acids as amphoteric molecules
Amino acids are amphoteric compounds. This is because they have an acidic group (COOH) and a basic group (NH2) – this means they can react with alkalines and acids to form salts.
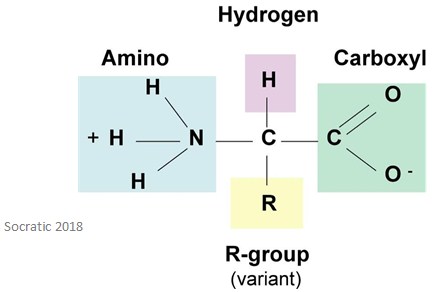
When in acid solutions, amino acids carry a positive charge. This means that in an alkaline solution, amino acids carry a negative charge. So, when an amino acid dissolves in water, it exists in a form where it carries both charges, much like the image above. This happens because the carboxyl group dissociates to release a proton, which passes to the amino group.
When amino acids possess both a positive and a negative charge at the same time it is called a zwitterion. These zwitterions can react with a base by liberating a proton from the amino group – it will then possess an overall negative charge. They can also react with an acid by combining with the proton of the acid – it will then possess an overall positive charge. Both of these zwitterion reactions are reversible. The structure of a zwitterion is shown below.
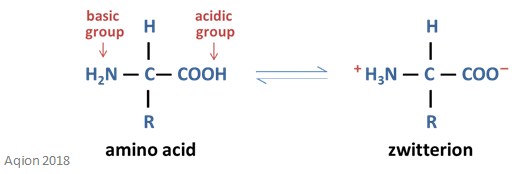
The pH at which the amino acid molecule possesses an overall charge of zero is called the isoelectric point (this can also be called the isoelectric pH).
Other examples of amphoteric molecules
Water, amino acids, hydrogen carbonate ions, and hydrogen sulphate ions are all amphoteric substances.
Many metal and metalloid oxides and hydroxides are amphoteric, such as aluminium, arsenic, chromium, cobalt, copper, gold, iron, lead, silver, tin, and zinc.
Further Reading





