Facts, Summary & Definition
- Ambidentate ligands are a type of ligand which can bond to the central atom in two places, but not at the same time
- SCN- and NO2- are good examples of ambidentate ligands
What are ambidentate ligands?
To understand ambidentate ligands, one must first understand what a ligand is. A ligand is a molecule or ion (a functional group) that can bind to a central metal atom (which can be in a zero, negative, or positive oxidation state) – this bonding usually involves the ligand donating one or more electron pairs. This means that ligands act as Lewis bases (because they donate a pair of electrons), and the central atom acts as a Lewis acid (because they accept a pair of electrons). All ligands must have at least one donor atom with an electron pair which can be used to form a covalent bond with the central atom.
The reaction between a central metal ion and a ligand can be explained more simply in the reaction below:
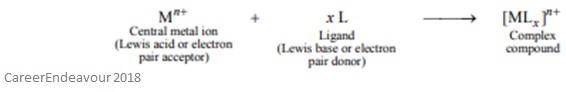
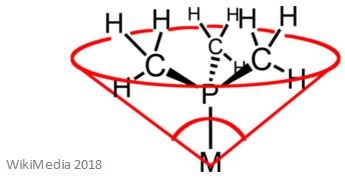
Ligands can be classified in a variety of ways such as: size, charge, and the number of electrons that are donated to the central metal ion. It is important to know that the size of a ligand is indicated by its cone angle. The cone angle of a ligand is the angle formed with the central metal atom – this is better explained by the image below.
Ligands also dictate the reactivity of the central metal atom when bound, including the reactivity of the ligands themselves and the ligand substitution rates.
Ambidentate ligands are ligands which can bond to the central atom in two places. This is because they have more than one donor atom which can coordinate.
It is important to note that these ligands are capable of bonding to a central atom through two different atoms, but only bonds with one of them at a time. This type of ligand also tends to be linear in geometry.
Examples of ambidentate ligands
SCN- is an example of an ambidentate ligand. This is because it can bond to a coordination centre through nitrogen as well as sulphur. The below image shows how SCN- can act as an ambidentate ligand.

As you can see, SCN- can bond either through the sulphur atom or the nitrogen atom, but not at the same time.
Another example of an ambidentate ligand is NO2-. This ion can bind to a central atom through either the nitrogen or one of the oxygen atoms, but again, not at the same time.
Linkage Isomerism
Linkage isomers are two (or more) compounds in which the donor atom is different (so, the connectivity between the atoms is different). Put more simply, the only difference between the two is what atoms in the ligand bind to the central ion.
This type of isomerism can therefore only occur when the compound contains an ambidentate ligand.
The image below shows linkage isomerism in the NO2 ligand. The two isomers have coordinated to a metal ion in two distinct ways.
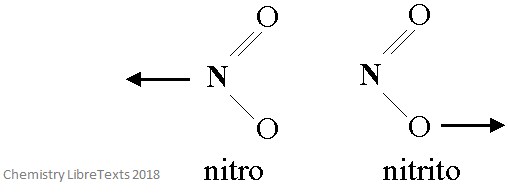
Different names are used to specify which atom has coordinated to the central metal ion. So, when NO2 binds with the N atom is it called nitro, but when it binds with the O atom it is called nitrito.
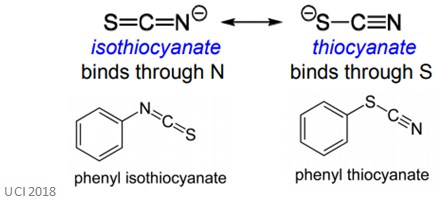
There are many other examples of this naming convention. For example, then SCN- binds through the lone pair of electrons on the S atom, the complexes are called thiocyanate. When the ligand binds through the N atom, the complexes are called isothiocyanate.
Further Reading
https://byjus.com/chemistry/ligands-complexity-of-coordination-compounds/





