Facts, Summary & Definition
- Affinity is the tendency of a chemical species to react with another species to form a chemical compound
- Chemical affinity depends on both stereochemistry and electrostatic interactions
- Electron affinity is the amount of energy released or spent when an electron in added to a neutral atom
- Drug affinity is how well a certain drug can bind to a receptor
What is affinity?
The term ‘affinity’ is the tendency of a chemical species (such as an atom or a molecule) to react with another species to form a chemical compound. There are multiple, more specific, uses of the term, such as chemical affinity and electron affinity.
What is chemical affinity?
Chemical affinity exclusively refers to the tendency of a chemical species to react with another. For example, we say that fluorine has a very high affinity for hydrogen. This is because the two species combine readily to form HF, as seen in this equation:
2F2 + 2H2O = 4HF + O2
Affinity can also be referred to as the tendency of certain atoms (or molecules) to aggregate or bond together. For example, a positively charged molecule will have affinity for a negatively charged molecule. The affinity of a chemical species depends on the stereochemistry and the electrostatic interactions.
Stereochemistry relates to every aspect of the spatial relationships of atoms within a molecule. An easy way to remember this is by knowing that the prefix ‘stereo’ means ‘3D’. This becomes especially important in biological systems, where molecules may have the same constituent atoms but the shape of the molecule is different – this is called a stereoisomer. Stereoisomers will, therefore, have different affinities for certain molecules.
Electrostatic interactions can also be called van der Waals forces – it is defined as the attraction or repulsion interactions between two molecules with electrical charges. An excellent example of this is found in the DNA double helix. The delta positive and delta negative ends of the polar N-H bond in DNA bases form an electrostatic attraction – this is shown in the diagram below.
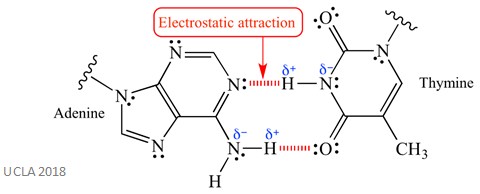
Van der Waals forces are a type of non-covalent molecular interaction. This is the type of molecular force which operate in liquid water. So, when these forces are broken (perhaps by heat), the water molecules no longer interact and form water vapour.
What is electron affinity?
Electron affinity, denoted as Eea, refers to the amount of energy released or spent when an electron is added to a neutral atom. The electron affinity is therefore a measure of the attraction between and incoming electron and the nucleus. So, the stronger the attraction, the more energy will be released.
This is in a gaseous state and forms a negative ion. It is shown in the following equation:
X + e− → X− + energy
Non-metal elements tend to have a higher electron affinity than metals in the periodic table. Electron affinities are used to develop and electronegativity scale for atoms.
The first electron affinity is defined as the amount of energy released when 1 mole of gaseous atom each gain an electron. They always have negatives values, as a negative value shows a release of energy, and the values are set. For example, the first electron affinity of bromine is -295 kJ mol-1.
The second electron affinity is the energy required to add a single electron to each ion in 1 mole of gaseous 1- ions to create 1 mole of gaseous 2- ions. This is only normally encountered in oxygen and sulphur (both group 6 elements). As you may suspect, this takes a lot of energy to do.
What is drug affinity?
In terms of pharmaceuticals, drug affinity is how well a certain drug can bind to its intended receptor. A faster or stronger binding means a higher affinity. It can also be defined as the measure of ‘tightness’ with which a drug can bind to its intended receptor.
In more statistical terms, it is the probability that a drug will bind to an available receptor at any given point in time.
In order for a drug to successfully bind to a receptor, the functional groups on the drug must interact with the receptor site. The affinity of a drug is determined by the number of bonds and the so-called ‘level of fit’ between the drug and its receptor. It is characterised by Ka – this is called the equilibrium constant.
It is important to note that in pharmacology, affinity is not the same as efficacy. Efficacy is defined as the ability of a drug to produce a maximum response. So, affinity is how likely a drug is to bind to a receptor, but the efficacy is what happens once that drug is bound. You will also need to remember that both agonists and antagonists have affinity for receptors.
Further Reading
http://www.eoht.info/page/Chemical+affinity





