Facts, Summary & Definition
- An adduct is a product formed by an addition reaction
- Adducts can only be formed from reactants which have multiple bonds, such as alkenes and carbonyl groups
- Adducts can be formed through two types of addition reaction: electrophilic and nucleophilic
- Adducts often form between Lewis acids and Lewis bases
What is an adduct?
An adduct is a product formed at the end of an addition reaction. This is a reaction in which two or more molecules react and combine to form one larger product. This type of reaction can only occur between chemical compounds which have multiple bonds – compounds like alkenes (double bonds) and alkynes (triple bonds). Carbonyl (C=O) and imine (C=N) groups can also undergo addition reactions, and thus form adducts.
How are adducts formed?
As mentioned before, adducts are formed through addition reactions. In this type of reaction, the double bond partially breaks when a reacting molecule attacks and adds on. The reaction below shows an addition reaction between ethene and bromine.
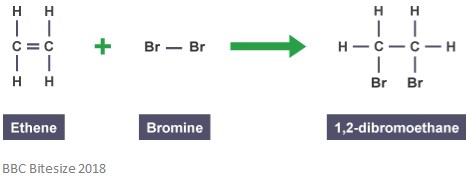
The adduct in this reaction is 1,2-dibromoethane. This is because it is a distinct species which contains all atoms of all the components.
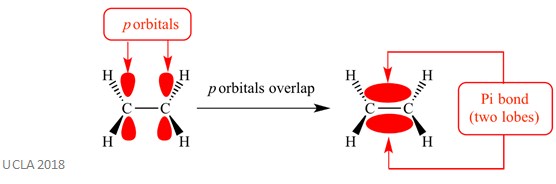
These addition reactions usually involve pi (π) bonds. This is a complex type of bond in which the p orbitals on adjacent atoms overlap – it is this overlapping which causes the pi bond. This occurs perpendicular to any sigma bonds between those two same atoms. This pi bond also has two orbital lobes – one is above the plane of the sigma bond, and the other is below the plane of the sigma bond. The pi bond is shown in the diagram below.
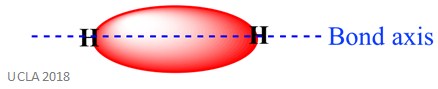
The same reaction can also involve sigma (σ) bonds. This is a type of covalent bond form by the overlap of atomic orbitals along the bond axis – in other words, along the line which connects the two bonded atoms. For example, the sigma bond in a hydrogen atom is formed by the overlap of two 1s orbitals – one from each hydrogen atom. This is better shown in the image below- the red area shown the sigma bond.
There are two main types of addition reaction: electrophilic addition and nucleophilic addition.
Electrophilic Addition
An electrophilic addition reaction is a type of reaction in which a bond is broken, and two new bonds are formed. As discussed earlier, the reactant must have a double or triple bond – the electrophile adds to a pi bond in this instance. This is an addition reaction, and so nothing is lost in the process – all of the atoms found in the reactant molecules are found in the adduct, too.
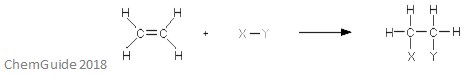
The above image shows the basic of an electrophilic addition reaction. As you can see, all of the atoms in the reactants are found in the adduct.
Nucleophilic Addition
A nucleophilic addition reaction is a type of reaction in which a double bond is broken, and two single bonds are formed. An electron-deficient (electrophilic) double or triple bond reacts with something which is electron-rich (nucleophile). The nucleophile adds to a pi bond in this instance. An example of a nucleophilic addition reaction is shown below.
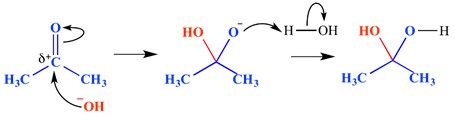
In this example, a hydroxide ion has added to the delta positive end of the carbonyl group – this has formed an oxyanionic tetrahedral intermediate molecule. The end result is, therefore, the addition of water across the carbonyl group pi bond.
Lewis acids and Lewis bases
Adducts are often known to form between Lewis acids and Lewis bases. The molecule formed is called a Lewis acid-base adduct (or a Lewis acid-base complex). As adducts are only formed through addition reactions (that is, without the simultaneous loss of a group), Lewis-acid and Lewis-base reactions cannot be that of the substitution kind.
A good example of this is the reaction between THF and BH3, which is shown in the image below. As you will see, these Lewis acid-Lewis base adducts are shown with dots – these represent electrons.
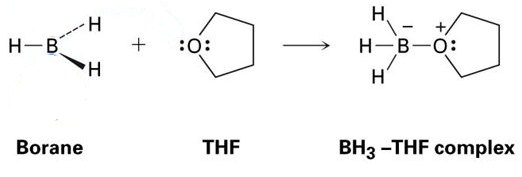
As you can see, the only product formed is the BH3-THF complex, which means it is an adduct.
These types of reactions can also be depicted with arrows – these arrows represent the donation of electrons from the base to the acid. The same reaction is shown below, but with arrows to indicate electron donation.
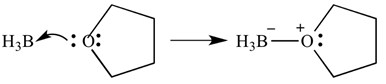
It is important to note here that these arrows have two ‘barbs’ on the pointed end. This indicates that a pair of electrons has been donated. If only one electron is donated, the arrow will only have ‘one’ bard on the pointed end.
In this instance, the Lewis acid is borane and the Lewis base is THF. As you can see, the base has donated a lone pair of electrons to the acid to form an oxygen-boron bond.
Compounds that are unable to form adducts because of the shape of the molecules are called frustrated Lewis pairs.
Further Reading
http://www.chem.ucla.edu/~harding/IGOC/L/lewis_acid_base_adduct.html
https://chem.libretexts.org/Ancillary_Materials/Reference/Organic_Chemistry_Glossary/Adduct





