Facts, Summary & Definition
- The Brønsted-Lowry theory is the most common theory of acidity
- Acidity is measured using the pH scale
- The pH of a solution can be measured by using an indicator or a pH meter
- Acids act as proton donors, and bases act as proton acceptors
- You can calculate the proton concentration if you know the pH of a solution
What is acidity?
There are two main theories of acidity: the Brønsted-Lowry theory and the Lewis theory.
The Brønsted-Lowry theory defines an acid as a proton (H+) donor, and therefore, bases are proton acceptors. This is the most useful current theory of acids and bases. For a compound to be able to act as a Brønsted-Lowry acid, it must have a hydrogen atom which it can lose but still remain somewhat stable. Some compounds can act as both acids and bases – such as water, because it can either lose a hydrogen atom to form OH- or gain one to form H3O+. These compounds are called amphiprotic.
The Lewis theory is a little more complicated and focuses on electron pairs. It defines an acid as an electron pair acceptor, and therefore, bases are electron pair donors. This definition is much broader than the Brønsted-Lowry theory because it includes compounds that, whilst they do not have protons, they still show acid/base behaviour.
How is acidity measured?
Acidity is quantitatively measured using the pH scale.
pH = -log10[H+(aq)]
As you can see from the image below, the lower the pH of a solution, the more acidic it is.
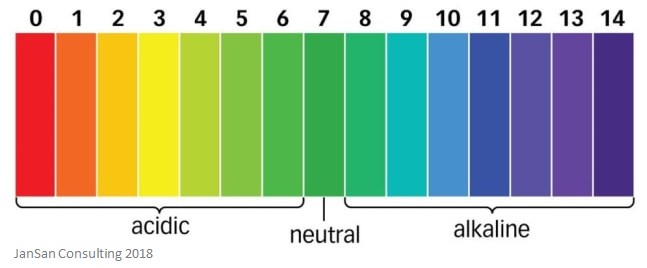
One important thing to remember is that, on the pH scale, the smaller the pH, the greater the concentration of protons. Also, a difference of one pH number means a tenfold difference in the number of protons – this means that a solution with a pH of 2 has ten times more protons that a solution of pH 3. This is described as a logarithmic scale.
pH can be measured using an indicator paper or an indicator solution (a very popular solution is called universal indicator). Such indicators are made from a mixture of different dyes that change colour when exposed to different proton concentrations. However, this method is not very precise, and so a pH meter can also be used. These have electrodes that dip into the solution and produce a voltage which is related to the proton concentration of the solution. This method is used to precisely and continuously measure pH levels.
Acidity of a compound
For Brønsted-Lowry acids, the acidity of a compound is described as the tendency to act as a proton donor. The acidity of one of these acids can be quantitatively expressed by the acid dissociation constant of the compound in water (or a different specified medium). The acid dissociation constant is a measure of the strength of an acid when it is in solution and is usually denoted as Ka.
Strong acids, such as nitric acid and sulfuric acid, fully dissociate in solution.
HA → H+ + A−
Weak acids, such as acetic acid, only partially dissociate in solution.
HA ⇌ H+ + A−
You can determine the acid dissociation constant of an acid through a method called titration. This method depends on an acid and base neutralising when in solution. A pH indicator is added to the sample, which indicates the neutralisation by changing colour. This type of technique is very useful in industry, for example, it is used to neutralise waste vegetable oil before processing – this removes the free fatty acids from the oil that would normally react to form soap.
Calculating proton concentration using pH
It is possible to calculate the concentration of protons in a solution if you know the pH of that solution.
Example: A solution has a pH of 3. Calculate the concentration of protons in the solution.
pH = -log10[H+(aq)]
3 = -log10[H+(aq)]
-3 = log10[H+(aq)]
[H+(aq)] = 1.0 x 10-3 mol dm-3
Acidity of a medium
This term is usually only used when a medium contains Brønsted-Lowry acids and is expressed by the appropriate acidity function.
Further Reading
https://www.chemguide.co.uk/physical/acidbaseeqia/acids.html





