Facts, Summary & Definition
- Light absorption is the process in which light is absorbed and converted into energy
- When electrons absorb energy, they become ‘excited’ and move to higher energy levels which are further away from the nucleus
- Electrons don’t like being in an excited state, and so fall back to their original energy level very quickly – they release a packet of energy called a photon when they do this
- Absorption and emission spectroscopy are two methods used to study
What is light absorption?
Light absorption is the process in which light is absorbed by matter and converted into energy. In an atom, electrons vibrate at a specific frequency – this is called the natural frequency. If a wave of light hits a material in which the electrons are vibrating at the same frequency as the wave of light, the electrons will absorb the energy and convert it into vibrational motion. This is why objects have different colours – different materials’ electrons will vibrate at different rates, and therefore absorb different frequencies of light.
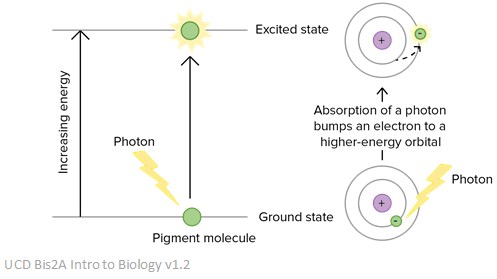
Light absorption and matter
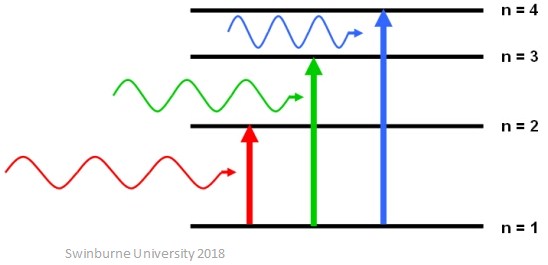
Electrons can only exist in discrete energy levels (these can also be called electron shells) – they can’t exist halfway between. The lowest energy level that an electron can be in is called the ground state. For an electron to move from a lower energy level to a higher energy level, it must absorb a set amount of energy because energy levels are quantised. This means that the energy absorbed by the electron must be exactly the same as the energy difference between the two levels.
When an electron absorbs energy, is it promoted to a higher energy level further away from the nucleus of the atom and is described as being ‘excited’.
What happens after an electron becomes excited?
Electrons don’t like being in an excited state. This means that after becoming excited and moving to a higher energy level, they soon fall back to their original energy level. However, to do this, they have to release a packet of energy – this is called a photon. The size of the photon released is exactly equal to the size of the jump the electron had to make in the first place.
Absorption Spectroscopy
Absorption spectroscopy is a technique used to measure the absorption of energy. The absorption spectrum of a certain material is shown by a continuous band of colour with black lines between them. The coloured parts represent the total light that is focused on the material. The black lines show an absence of this light – these are the parts of the spectrum where the electrons have absorbed the light photons.

There are two types of absorption spectroscopy: atomic and molecular. Atomic absorption spectroscopy is the method of producing a spectrum when free atoms absorb different wavelengths of light – this is usually used for gases. Molecular absorption spectroscopy is the method of producing a spectrum when whole molecules absorb different wavelengths of light (usually ultraviolet or visible).
Absorption spectrums are the exact opposite of emission spectrums.
Emission Spectroscopy
Emission spectroscopy is used to measure the photons released when an electron falls to a lower energy level after becoming excited. The emission spectrum of a certain material is shown by a black band with separated coloured lines. These colours lines are the parts of the spectrum where photons have been released from the electrons when they fall to a lower energy level.

There are two types of emission spectroscopy: line and continuous. When the spectrum is shown as lots of lines separated by black spaces, it is a line emission spectrum. When the spectrum is shown as lots of colours in one particular wavelength, it is a continuous emission spectrum. Emission spectroscopy is used to identify a substance because the energy released when the electrons fall back to their ground state is different for every substance.
Emission spectrums are the exact opposite of absorption spectrums.
Further Reading
http://astronomy.swin.edu.au/cosmos/a/absorption+line





